What is the coulomb’s law – What is Coulomb’s Law? This fundamental law in electromagnetism governs the attractive or repulsive forces between charged objects. Imagine two tiny charged particles, like electrons, sitting in space. Coulomb’s Law tells us exactly how strongly they will push or pull on each other, depending on their charges and how far apart they are. This simple yet powerful law underpins countless phenomena, from the behavior of atoms to the workings of everyday devices.
In essence, Coulomb’s Law states that the electrostatic force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This means that stronger charges exert a greater force, and as the distance between the charges increases, the force weakens rapidly. This inverse square relationship is a recurring theme in physics, found in gravity and light as well.
Introduction to Coulomb’s Law
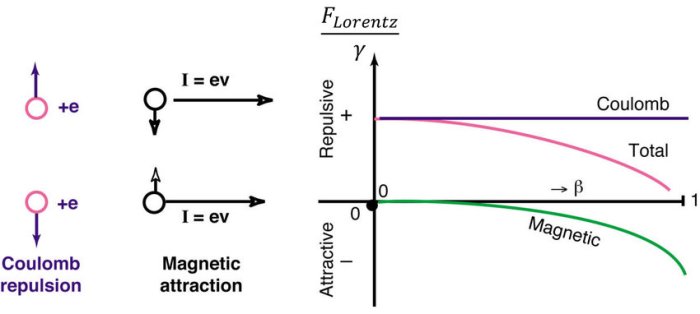
Coulomb’s Law is a fundamental principle in electromagnetism that describes the force of interaction between stationary electrically charged objects. It is named after Charles-Augustin de Coulomb, a French physicist who experimentally determined this law in the late 18th century. His meticulous experiments using a torsion balance provided a quantitative understanding of the forces between charges, paving the way for the development of modern electromagnetism.
Coulomb’s Law is essential for understanding a wide range of phenomena, from the behavior of atoms and molecules to the operation of electrical devices. It forms the basis for many important concepts in physics, such as electric fields, potential, and capacitance.
Mathematical Expression of Coulomb’s Law
Coulomb’s Law states that the force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. The force is attractive if the charges are of opposite signs and repulsive if they are of the same sign.
Mathematically, Coulomb’s Law can be expressed as:
F = k * (q1 * q2) / r^2
Where:
* F is the electrostatic force between the two charges.
* k is Coulomb’s constant, which has a value of approximately 8.98755 × 10^9 N⋅m^2/C^2.
* q1 and q2 are the magnitudes of the two charges.
* r is the distance between the two charges.
The unit of charge is the Coulomb (C).
Key Concepts and Definitions
Coulomb’s Law is a fundamental principle in electromagnetism that describes the force of interaction between electrically charged objects. To understand this law, it is essential to grasp the concepts of electric charge, electric force, and electric field.
Electric Charge
Electric charge is a fundamental property of matter that describes its ability to interact with electromagnetic forces. It is a quantized property, meaning it exists in discrete units called elementary charges. The fundamental unit of electric charge is the charge of an electron, which is approximately -1.602 x 10^-19 Coulombs.
Types of Electric Charges
There are two types of electric charges: positive and negative.
- Positive Charges: These charges are associated with the presence of protons, which are found in the nucleus of an atom.
- Negative Charges: These charges are associated with the presence of electrons, which orbit the nucleus of an atom.
Opposite charges attract each other, while like charges repel. For instance, two protons will repel each other, while a proton and an electron will attract each other. This interaction between charges forms the basis of Coulomb’s Law.
Electric Force, What is the coulomb’s law
The electric force is the force of attraction or repulsion between charged objects. It is a fundamental force of nature, along with the gravitational force, the weak force, and the strong force. The electric force is responsible for a wide range of phenomena, including the binding of atoms into molecules, the behavior of electrical circuits, and the interaction of charged particles in particle accelerators.
Electric Field
An electric field is a region of space where an electric charge would experience a force. It is a vector quantity, meaning it has both magnitude and direction. The direction of the electric field at a point is defined as the direction of the force that would be exerted on a positive test charge placed at that point. The magnitude of the electric field is defined as the force per unit charge.
The electric field is a powerful concept that helps us understand the behavior of electric charges in space. It allows us to visualize the influence of charges on their surroundings without explicitly considering the presence of other charges.
Closing Notes

Coulomb’s Law provides a fundamental understanding of electrostatic interactions, a cornerstone of electromagnetism. It elegantly explains the forces between charged particles, laying the groundwork for more complex phenomena. From the workings of everyday devices like photocopiers and ink-jet printers to the intricate dance of atoms in molecules, Coulomb’s Law plays a vital role. Its influence extends far beyond the realm of classical physics, serving as a foundation for more advanced theories like quantum electrodynamics (QED), which describes the interaction of light and matter at the most fundamental level.
Top FAQs: What Is The Coulomb’s Law
What are some real-world examples of Coulomb’s Law in action?
Coulomb’s Law is at play in various technologies, including electrostatic precipitators that remove dust and pollutants from air, photocopiers that use static electricity to transfer toner to paper, and ink-jet printers that rely on charged ink droplets.
How does Coulomb’s Law relate to the concept of electric field?
The electric field is a region of space where a charged object experiences a force. Coulomb’s Law helps define the strength of the electric field created by a point charge.
Is Coulomb’s Law always accurate?
While Coulomb’s Law is highly accurate for macroscopic charges at typical distances, it breaks down at extremely small distances, where quantum effects become significant.