How do you find the rate law sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. Understanding the rate law of a chemical reaction is essential for predicting how fast a reaction will proceed and for optimizing reaction conditions. It’s like having a roadmap for a chemical journey, guiding us to predict the speed and efficiency of the transformation. This knowledge allows us to control the pace of chemical reactions, ensuring that they happen at the desired rate and yield the desired products.
The rate law is a mathematical expression that describes the relationship between the rate of a reaction and the concentrations of the reactants. It’s like a secret code that reveals the hidden dynamics of a chemical reaction. We can unravel this code by conducting experiments and analyzing the data, using techniques like the method of initial rates or integrated rate laws. This process is akin to deciphering an ancient text, uncovering the fundamental principles that govern the behavior of chemical reactions.
Introduction to Rate Laws
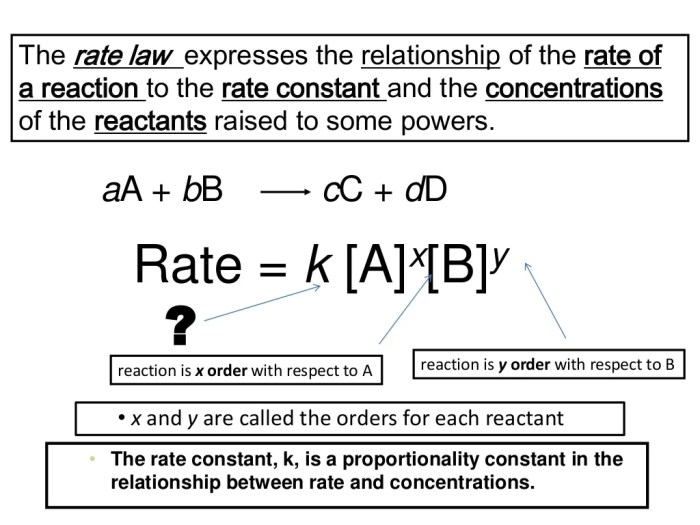
In the realm of chemical kinetics, understanding the speed at which chemical reactions occur is paramount. This is where rate laws come into play. A rate law is a mathematical expression that describes the relationship between the rate of a reaction and the concentrations of the reactants. It essentially quantifies how fast a reaction proceeds under specific conditions.
Understanding rate laws is crucial for various reasons. It allows us to predict how the rate of a reaction will change under different conditions, such as altering the concentration of reactants or changing the temperature. This knowledge is invaluable for optimizing reaction conditions, controlling the rate of reactions, and designing efficient chemical processes. Moreover, rate laws provide insights into the mechanism of a reaction, helping us understand the step-by-step process by which reactants transform into products.
Types of Rate Laws
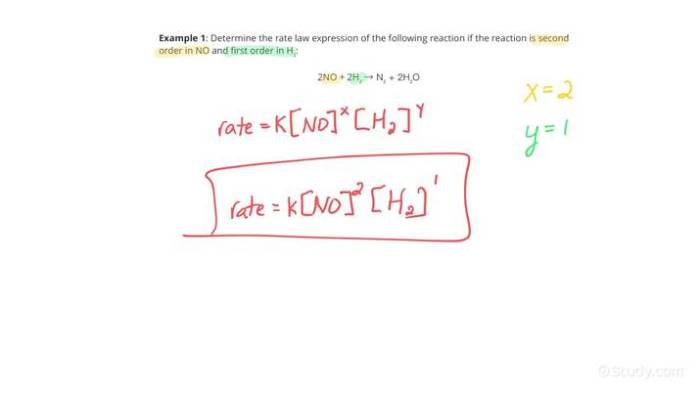
Rate laws can be categorized based on the order of the reaction. The order of a reaction refers to the power to which the concentration of each reactant is raised in the rate law.
Here are some common types of rate laws:
* Zero-order reactions: The rate of a zero-order reaction is independent of the concentration of the reactants.
* The rate law is expressed as:
*
Rate = k
* where k is the rate constant.
* Examples include the decomposition of N2O on a hot platinum surface.
* First-order reactions: The rate of a first-order reaction is directly proportional to the concentration of one reactant.
* The rate law is expressed as:
*
Rate = k[A]
* where k is the rate constant and [A] is the concentration of reactant A.
* Examples include the radioactive decay of isotopes, such as the decay of carbon-14.
* Second-order reactions: The rate of a second-order reaction is proportional to the square of the concentration of one reactant or the product of the concentrations of two reactants.
* The rate law is expressed as:
*
Rate = k[A]2
* or
*
Rate = k[A][B]
* where k is the rate constant, [A] and [B] are the concentrations of reactants A and B, respectively.
* Examples include the reaction between hydrogen and iodine to form hydrogen iodide.
* Third-order reactions: The rate of a third-order reaction is proportional to the cube of the concentration of one reactant or the product of the concentrations of three reactants.
* The rate law is expressed as:
*
Rate = k[A]3
* or
*
Rate = k[A]2[B]
* or
*
Rate = k[A][B][C]
* where k is the rate constant, [A], [B], and [C] are the concentrations of reactants A, B, and C, respectively.
* Examples include the reaction between nitric oxide and ozone.
Determining the Rate Law Experimentally: How Do You Find The Rate Law
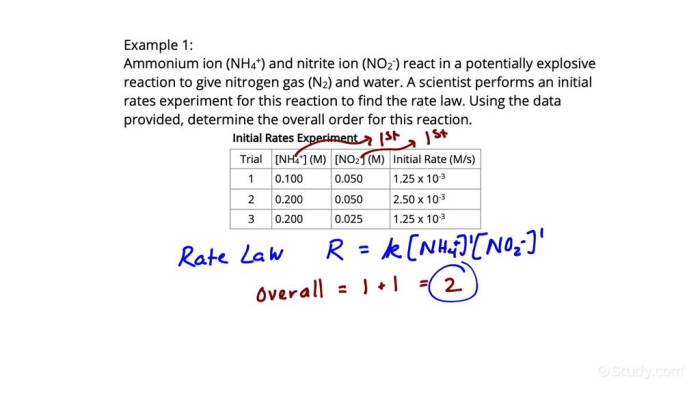
The rate law, which describes the relationship between the rate of a reaction and the concentrations of reactants, can be determined experimentally. One common method is the method of initial rates. This method involves measuring the initial rates of a reaction at different initial concentrations of reactants, while keeping the temperature constant.
Method of Initial Rates
The method of initial rates is a powerful technique for determining the order of a reaction. This method involves measuring the initial rates of a reaction at different initial concentrations of reactants, while keeping the temperature constant. By comparing the initial rates at different concentrations, we can deduce the order of the reaction with respect to each reactant.
- Procedure: The method of initial rates involves performing a series of experiments where the initial concentrations of the reactants are varied systematically. For each experiment, the initial rate of the reaction is measured. The initial rate is the rate of the reaction at the very beginning of the reaction, before any significant amount of product has formed.
- Analysis: Once the initial rates are measured for different initial concentrations, the order of the reaction with respect to each reactant can be determined by comparing the initial rates. For example, if doubling the concentration of a reactant doubles the initial rate, the reaction is first order with respect to that reactant. If doubling the concentration of a reactant quadruples the initial rate, the reaction is second order with respect to that reactant.
Determining the Order of a Reaction
The order of a reaction is the sum of the exponents in the rate law. It tells us how the rate of a reaction changes as the concentrations of the reactants change. The order of a reaction can be determined experimentally using the method of initial rates.
- First-order Reactions: For a first-order reaction, the rate is directly proportional to the concentration of the reactant. This means that doubling the concentration of the reactant will double the rate of the reaction.
- Second-order Reactions: For a second-order reaction, the rate is proportional to the square of the concentration of the reactant. This means that doubling the concentration of the reactant will quadruple the rate of the reaction.
- Zero-order Reactions: For a zero-order reaction, the rate is independent of the concentration of the reactant. This means that changing the concentration of the reactant will not affect the rate of the reaction.
Limitations of the Method of Initial Rates
While the method of initial rates is a valuable tool for determining the rate law, it has some limitations.
- Assumption of Initial Rate: The method of initial rates assumes that the initial rate of the reaction is a good approximation of the average rate of the reaction over a short period of time. This assumption may not be valid for reactions that are very slow or very fast.
- Side Reactions: The method of initial rates assumes that there are no side reactions occurring. If side reactions are present, they can affect the measured initial rates and make it difficult to determine the true rate law.
- Complex Reactions: The method of initial rates may not be suitable for complex reactions that involve multiple steps. In these cases, it may be difficult to isolate the rate law for a single step in the reaction mechanism.
Integrated Rate Laws
The differential rate laws we discussed earlier provide a snapshot of the reaction rate at a specific moment. However, they don’t tell us how the concentration of reactants changes over time. This is where integrated rate laws come in. Integrated rate laws are mathematical expressions that relate the concentration of reactants to time. They are derived from the differential rate laws by integrating them with respect to time.
Integrated rate laws are useful because they allow us to:
* Predict the concentration of reactants or products at any given time.
* Determine the rate constant of a reaction.
* Calculate the half-life of a reaction.
Integrated Rate Laws for Different Reaction Orders
Integrated rate laws take different forms depending on the order of the reaction. Let’s examine the most common cases:
Zero-Order Reactions
For a zero-order reaction, the rate is independent of the concentration of the reactant. The integrated rate law for a zero-order reaction is:
[A]t = -kt + [A]0
Where:
* [A]t is the concentration of reactant A at time t.
* [A]0 is the initial concentration of reactant A.
* k is the rate constant.
This equation is in the form of a straight line (y = mx + c), with [A]t on the y-axis and t on the x-axis. The slope of the line is -k, and the y-intercept is [A]0.
First-Order Reactions
In a first-order reaction, the rate is directly proportional to the concentration of the reactant. The integrated rate law for a first-order reaction is:
ln[A]t = -kt + ln[A]0
This equation is also in the form of a straight line, with ln[A]t on the y-axis and t on the x-axis. The slope of the line is -k, and the y-intercept is ln[A]0.
Second-Order Reactions
For a second-order reaction, the rate is proportional to the square of the concentration of the reactant. The integrated rate law for a second-order reaction is:
1/[A]t = kt + 1/[A]0
Again, this equation is in the form of a straight line, with 1/[A]t on the y-axis and t on the x-axis. The slope of the line is k, and the y-intercept is 1/[A]0.
Determining the Rate Constant and Half-Life
The integrated rate laws can be used to determine the rate constant (k) and half-life (t1/2) of a reaction.
Rate Constant
The rate constant can be determined from the slope of the integrated rate law plot. For example, for a first-order reaction, the rate constant is equal to the negative of the slope of the ln[A]t vs. t plot.
Half-Life
The half-life of a reaction is the time it takes for the concentration of the reactant to decrease to half its initial value. The half-life can be calculated from the integrated rate law. For example, for a first-order reaction, the half-life is given by:
t1/2 = 0.693/k
This equation shows that the half-life of a first-order reaction is independent of the initial concentration.
Factors Affecting Rate Laws
The rate law, which describes the relationship between reactant concentrations and reaction rate, is not fixed and can be influenced by various factors. Understanding these factors allows us to control and predict reaction rates, which is crucial in various fields like chemical engineering, biochemistry, and materials science.
Temperature
Temperature significantly affects reaction rates. As temperature increases, molecules move faster, resulting in more frequent and energetic collisions. This increased collision frequency and energy lead to a higher probability of successful collisions, thus accelerating the reaction.
The relationship between temperature and rate constant is described by the Arrhenius equation:
k = A * exp(-Ea/RT)
where:
* k is the rate constant
* A is the pre-exponential factor (frequency factor)
* Ea is the activation energy
* R is the ideal gas constant
* T is the absolute temperature
The Arrhenius equation reveals that the rate constant increases exponentially with temperature. The activation energy (Ea) represents the minimum energy required for reactants to overcome the energy barrier and form products.
Catalysts, How do you find the rate law
Catalysts are substances that accelerate reaction rates without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. By lowering the activation energy, catalysts increase the fraction of molecules possessing sufficient energy to react, thus increasing the reaction rate.
For example, in the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2), the reaction is slow at room temperature. However, adding a catalyst like manganese dioxide (MnO2) significantly speeds up the decomposition, producing oxygen bubbles rapidly.
Surface Area
The surface area of reactants can significantly impact reaction rates, especially in heterogeneous reactions involving reactants in different phases (e.g., solid-liquid or solid-gas). A larger surface area provides more contact points for the reactants, leading to more frequent collisions and a faster reaction rate.
For instance, a lump of sugar dissolves slowly in water compared to powdered sugar. The powdered sugar has a much larger surface area exposed to the water, leading to faster dissolution.
Concentration
The concentration of reactants directly influences reaction rates. As the concentration of reactants increases, the frequency of collisions between reactant molecules increases, leading to a faster reaction rate.
For example, burning a piece of paper in air is a relatively slow process. However, if the paper is placed in a pure oxygen environment, it burns much faster. The higher concentration of oxygen molecules increases the collision frequency and reaction rate.
Applications of Rate Laws
Rate laws are not merely theoretical constructs; they have profound implications in various fields, offering a powerful tool for predicting reaction outcomes, optimizing reaction conditions, and understanding the intricacies of chemical processes.
Predicting Reaction Outcomes
Rate laws provide a framework for predicting the extent to which a reaction will proceed and the time it takes to reach a particular point of completion. The rate constant, a key component of the rate law, reflects the intrinsic speed of the reaction. By knowing the rate law and the initial concentrations of reactants, we can calculate the concentration of reactants and products at any given time. For instance, consider a reaction with a known rate law and rate constant. If we start with a specific concentration of reactants, the rate law allows us to determine the time required for half of the reactants to be consumed (half-life) or the time required for the reaction to reach 90% completion.
Optimizing Reaction Conditions
Rate laws provide valuable insights into optimizing reaction conditions to maximize product yield or minimize reaction time. For example, if a reaction exhibits a high activation energy, we can increase the reaction rate by elevating the temperature. Similarly, if the reaction is sensitive to the concentration of a particular reactant, we can manipulate its concentration to achieve desired outcomes.
- Temperature: Increasing temperature generally increases the rate of reaction. This is because higher temperatures lead to more frequent collisions between molecules, and a higher proportion of these collisions have sufficient energy to overcome the activation energy barrier.
- Concentration: The rate of a reaction is typically proportional to the concentration of reactants. Increasing the concentration of reactants leads to more frequent collisions, resulting in a faster reaction rate.
- Catalyst: Catalysts provide an alternative reaction pathway with a lower activation energy, effectively speeding up the reaction without being consumed in the process.
Real-World Applications
Rate laws have widespread applications in various fields:
- Chemical Engineering: Rate laws are essential for designing and optimizing chemical reactors, predicting reaction rates, and determining the time required for reactions to reach completion.
- Pharmaceuticals: In pharmaceutical research, rate laws are used to study drug degradation and predict shelf life. Understanding the rate of drug decomposition is crucial for ensuring drug stability and efficacy.
- Environmental Science: Rate laws play a crucial role in understanding the kinetics of environmental processes, such as the degradation of pollutants and the formation of atmospheric ozone.
- Materials Science: Rate laws are employed to study the growth of crystals, the formation of thin films, and the corrosion of metals.
Summary
By understanding the rate law, we gain a powerful tool for manipulating and controlling chemical reactions. We can predict reaction outcomes, optimize reaction conditions, and even design new reactions. The applications of rate laws are vast, spanning from the development of new drugs and materials to the optimization of industrial processes. It’s a fascinating journey that takes us from the microscopic world of molecules to the macroscopic world of real-world applications, revealing the interconnectedness of chemistry and our everyday lives.
Clarifying Questions
What are the units of the rate constant?
The units of the rate constant depend on the overall order of the reaction. For example, for a first-order reaction, the rate constant has units of s-1, while for a second-order reaction, the rate constant has units of M-1s-1.
How can I determine the order of a reaction experimentally?
The order of a reaction can be determined experimentally by using the method of initial rates. This involves measuring the initial rate of the reaction at different concentrations of reactants and then analyzing the data to see how the rate changes with concentration.
What is the difference between a rate law and an integrated rate law?
A rate law describes the relationship between the rate of a reaction and the concentrations of reactants at a given instant in time. An integrated rate law relates the concentrations of reactants to time.