What is Boyle’s Law? It’s a fundamental principle in physics and chemistry that describes the relationship between the pressure and volume of a gas. Imagine a balloon: as you blow into it, the volume increases, and the pressure inside also rises. Boyle’s Law explains this phenomenon, and it has far-reaching implications in various fields, from medicine to engineering.
Robert Boyle, an Irish chemist and physicist, discovered this law in the 17th century. He meticulously conducted experiments with air, meticulously measuring the changes in pressure and volume. His findings led to the formulation of Boyle’s Law, which states that for a fixed mass of gas at a constant temperature, the pressure and volume are inversely proportional. This means that if you increase the pressure on a gas, its volume will decrease proportionally, and vice versa.
Introduction to Boyle’s Law

Boyle’s Law is a fundamental principle in chemistry and physics that describes the relationship between the pressure and volume of a gas at constant temperature. This law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume when the temperature and the amount of gas are held constant.
Historical Context and Robert Boyle
Robert Boyle, an Irish chemist and physicist, is credited with the discovery of Boyle’s Law in 1662. He conducted a series of experiments using a J-shaped tube filled with mercury and air, which he meticulously documented in his book, “New Experiments Physico-Mechanical, Touching the Spring of the Air and its Effects.” In his experiments, Boyle observed that as the pressure on the air in the tube increased, the volume of the air decreased proportionally. This relationship was later formalized into Boyle’s Law.
Significance of Boyle’s Law
Boyle’s Law has played a crucial role in advancing our understanding of gases and their behavior. It has numerous applications in various fields, including:
- Chemistry: Boyle’s Law is essential for understanding the behavior of gases in chemical reactions and for calculating the volume or pressure of gases involved in reactions. It also helps in determining the molar mass of gases.
- Physics: Boyle’s Law is fundamental in the study of thermodynamics and fluid mechanics. It is used to explain the behavior of gases in engines, pumps, and other devices that involve gas compression or expansion.
- Engineering: Engineers use Boyle’s Law to design and analyze systems that involve gases, such as air compressors, vacuum systems, and gas pipelines.
- Medicine: Boyle’s Law is applied in medical equipment such as ventilators and oxygen tanks, which rely on the relationship between pressure and volume to deliver gases to patients.
Mathematical Representation of Boyle’s Law
Boyle’s Law, a fundamental principle in chemistry and physics, can be expressed mathematically, providing a precise way to understand the relationship between pressure and volume of a gas. This mathematical representation allows us to calculate pressure or volume changes under specific conditions, making it a powerful tool for various applications.
Formula and Variables
The mathematical formula for Boyle’s Law is:
P₁V₁ = P₂V₂
Where:
* P₁ represents the initial pressure of the gas.
* V₁ represents the initial volume of the gas.
* P₂ represents the final pressure of the gas.
* V₂ represents the final volume of the gas.
This formula highlights the inverse relationship between pressure and volume: as pressure increases, volume decreases, and vice versa, while the temperature remains constant.
Using the Formula
The formula can be used to calculate either pressure or volume if the other three values are known. For example:
* Calculating Pressure: If we know the initial pressure (P₁), initial volume (V₁), and final volume (V₂), we can calculate the final pressure (P₂) using the formula:
P₂ = (P₁V₁) / V₂
* Calculating Volume: Similarly, if we know the initial pressure (P₁), initial volume (V₁), and final pressure (P₂), we can calculate the final volume (V₂) using the formula:
V₂ = (P₁V₁) / P₂
Numerical Examples
To illustrate the application of Boyle’s Law, let’s consider a few numerical examples:
Example 1: A gas occupies a volume of 5 liters at a pressure of 2 atmospheres. If the pressure is increased to 4 atmospheres, what is the final volume?
Solution:
* P₁ = 2 atmospheres
* V₁ = 5 liters
* P₂ = 4 atmospheres
* V₂ = ?
Using the formula, we can calculate V₂:
V₂ = (P₁V₁) / P₂ = (2 atmospheres * 5 liters) / 4 atmospheres = 2.5 liters
Therefore, the final volume of the gas is 2.5 liters.
Example 2: A gas occupies a volume of 10 liters at a pressure of 1.5 atmospheres. If the volume is reduced to 5 liters, what is the final pressure?
Solution:
* P₁ = 1.5 atmospheres
* V₁ = 10 liters
* V₂ = 5 liters
* P₂ = ?
Using the formula, we can calculate P₂:
P₂ = (P₁V₁) / V₂ = (1.5 atmospheres * 10 liters) / 5 liters = 3 atmospheres
Therefore, the final pressure of the gas is 3 atmospheres.
These examples demonstrate how Boyle’s Law can be used to predict the behavior of gases under changing pressure and volume conditions.
Applications of Boyle’s Law
Boyle’s Law, a fundamental principle in physics, finds numerous applications in various fields, influencing our daily lives and impacting technological advancements. This law, which describes the inverse relationship between the pressure and volume of a gas at a constant temperature, plays a crucial role in various phenomena and processes.
Applications in Medicine
Boyle’s Law plays a vital role in understanding and managing respiratory processes. It explains how the lungs expand and contract during breathing, enabling the intake and expulsion of air.
- Breathing: During inhalation, the diaphragm contracts and the chest cavity expands, decreasing the pressure inside the lungs. This pressure difference between the atmosphere and the lungs causes air to flow into the lungs. Conversely, during exhalation, the diaphragm relaxes, the chest cavity contracts, increasing the pressure inside the lungs, forcing air out. This cyclical process, driven by pressure changes governed by Boyle’s Law, allows for the efficient exchange of gases in the lungs.
- Scuba Diving: Scuba divers must understand Boyle’s Law to ensure safe underwater excursions. As a diver descends, the pressure surrounding them increases. This increased pressure compresses the air in their scuba tanks, reducing the volume of air available for breathing. Divers need to adjust their breathing rate and depth to compensate for these pressure changes. They also need to be aware of the potential dangers of air embolism, which can occur if a diver ascends too quickly, causing the air in their lungs to expand rapidly due to decreasing pressure.
Applications in Engineering
Boyle’s Law finds extensive applications in various engineering fields, particularly in the design and operation of hydraulic and pneumatic systems.
- Hydraulic Systems: Hydraulic systems utilize the incompressibility of liquids to transmit force. These systems, often used in heavy machinery and construction equipment, rely on the principle that pressure applied to an enclosed fluid is transmitted equally throughout the fluid. Boyle’s Law plays a role in understanding how pressure changes affect the volume of fluid in hydraulic systems, ensuring efficient force transmission.
- Pneumatic Devices: Pneumatic systems use compressed air to power various tools and equipment. Boyle’s Law governs the behavior of compressed air in these systems, determining the force and speed of pneumatic actuators. The relationship between pressure and volume is crucial for designing and operating pneumatic systems effectively.
Applications in Everyday Life
Boyle’s Law is also present in everyday objects and activities, often without us even realizing it.
- Bicycle Pumps: When you use a bicycle pump to inflate a tire, you are applying Boyle’s Law. The pump compresses air, increasing its pressure and reducing its volume. This compressed air is then transferred to the tire, where it expands and inflates the tire. The pump leverages the inverse relationship between pressure and volume to efficiently inflate tires.
- Balloons: Inflating a balloon is another example of Boyle’s Law in action. As you blow air into a balloon, you increase the pressure inside the balloon. This increased pressure causes the balloon to expand, increasing its volume. The balloon stretches to accommodate the increased volume of air, demonstrating the inverse relationship between pressure and volume.
Limitations and Considerations
While Boyle’s Law is a fundamental principle in understanding gas behavior, it’s important to acknowledge its limitations and the conditions under which it may not hold true. The law is based on the ideal gas model, which assumes that gas molecules have negligible volume and do not interact with each other. In reality, these assumptions break down under certain conditions, leading to deviations from Boyle’s Law.
Ideal Gas Behavior
The concept of ideal gas behavior is crucial for understanding the limitations of Boyle’s Law. An ideal gas is a theoretical model that assumes gas molecules:
- Have negligible volume compared to the volume of the container they occupy.
- Do not interact with each other, except through perfectly elastic collisions.
- Follow the laws of classical mechanics.
Real gases, however, deviate from ideal gas behavior, particularly at high pressures and low temperatures. In these conditions, the volume of gas molecules becomes significant, and intermolecular forces become more prominent, leading to deviations from Boyle’s Law.
Conditions Under Which Boyle’s Law May Not Hold True
- High Pressure: At high pressures, the volume of gas molecules becomes a significant fraction of the total volume, invalidating the assumption of negligible volume in the ideal gas model. This leads to deviations from Boyle’s Law, as the actual volume occupied by the gas becomes greater than predicted.
- Low Temperature: At low temperatures, intermolecular forces become more significant. These forces can cause gas molecules to attract each other, reducing the effective volume available for expansion and leading to deviations from Boyle’s Law.
- Non-Ideal Gases: Gases that exhibit strong intermolecular forces, such as polar molecules, deviate significantly from ideal gas behavior. These forces can cause significant deviations from Boyle’s Law, especially at low temperatures and high pressures.
Examples of Situations Where Boyle’s Law Might Not Accurately Predict the Behavior of Gases
- High-pressure environments: In deep-sea diving, where the pressure is extremely high, the behavior of gases deviates significantly from Boyle’s Law. The increased pressure compresses the gas molecules, leading to a greater reduction in volume than predicted by the law. This is a critical factor in decompression sickness, a condition that can occur when divers ascend too quickly, causing dissolved gases in their blood to form bubbles.
- Low-temperature environments: In cryogenic applications, where gases are cooled to extremely low temperatures, intermolecular forces become dominant, leading to deviations from Boyle’s Law. For example, the behavior of gases in liquid nitrogen storage tanks may not be accurately predicted by Boyle’s Law due to the significant intermolecular interactions at these low temperatures.
Visual Representation of Boyle’s Law: What Is Boyle’s Law

Boyle’s Law, which describes the inverse relationship between pressure and volume of a gas at constant temperature, can be visually represented using graphs and diagrams. These representations offer a clear and intuitive understanding of how these two variables interact.
Graphing Pressure and Volume
Graphs are powerful tools for visualizing the relationship between two variables. In the case of Boyle’s Law, we can plot pressure (P) on the y-axis and volume (V) on the x-axis. The resulting graph will show a curve that reflects the inverse relationship between pressure and volume.
- Hyperbolic Curve: The graph of Boyle’s Law is a hyperbola. This shape signifies that as pressure increases, volume decreases proportionally, and vice versa. The curve gets progressively closer to the x-axis and y-axis but never actually touches them.
- Constant Temperature: It’s important to remember that Boyle’s Law holds true only at constant temperature. Any change in temperature would alter the relationship between pressure and volume, resulting in a different curve on the graph.
Diagram of a Closed System
To further illustrate Boyle’s Law, consider a closed system containing a gas. This system could be a sealed container like a syringe or a balloon.
- Initial State: In the initial state, the gas occupies a specific volume at a certain pressure.
- Decreasing Volume: If we reduce the volume of the container (e.g., pushing the plunger of a syringe), the gas molecules will be confined to a smaller space. This increased crowding leads to more frequent collisions between the molecules and the container walls.
- Increased Pressure: These more frequent collisions result in an increase in pressure. This demonstrates the inverse relationship described by Boyle’s Law – as volume decreases, pressure increases.
- Increasing Volume: Conversely, if we increase the volume of the container (e.g., pulling the plunger of a syringe), the gas molecules have more space to move around. This leads to fewer collisions with the container walls, resulting in a decrease in pressure.
Boyle’s Law states that the product of pressure and volume remains constant for a fixed mass of gas at a constant temperature.
Boyle’s Law and Other Gas Laws
Boyle’s Law is one of several fundamental laws that describe the behavior of ideal gases. While Boyle’s Law focuses on the relationship between pressure and volume, other laws, such as Charles’ Law and Gay-Lussac’s Law, explore the relationship between volume and temperature, and pressure and temperature, respectively. These laws, collectively known as the gas laws, provide a comprehensive understanding of how gases behave under various conditions.
Comparison of Gas Laws, What is boyle’s law
The following table summarizes the key features of Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law:
| Law | Relationship | Mathematical Representation | Conditions |
|---|---|---|---|
| Boyle’s Law | Inversely proportional relationship between pressure and volume | P1V1 = P2V2 | Constant temperature |
| Charles’ Law | Directly proportional relationship between volume and temperature | V1/T1 = V2/T2 | Constant pressure |
| Gay-Lussac’s Law | Directly proportional relationship between pressure and temperature | P1/T1 = P2/T2 | Constant volume |
Combined Gas Law
These individual gas laws can be combined into a single equation known as the Combined Gas Law, which expresses the relationship between pressure, volume, and temperature for a fixed amount of gas.
The Combined Gas Law: (P1V1)/T1 = (P2V2)/T2
The Combined Gas Law allows us to calculate the change in one variable (pressure, volume, or temperature) when the other two variables change. For example, if we know the initial pressure, volume, and temperature of a gas and the final pressure and volume, we can use the Combined Gas Law to calculate the final temperature.
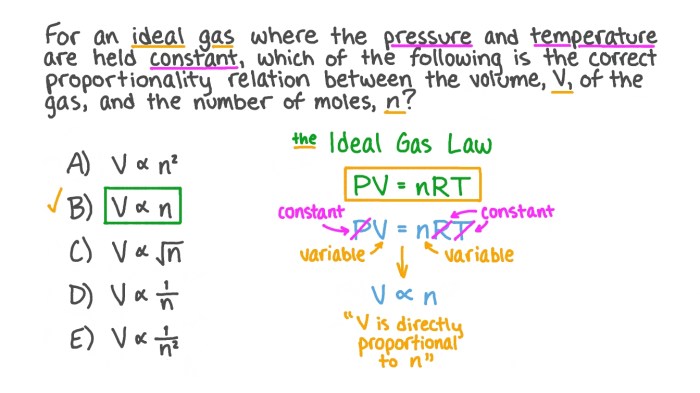
Ideal Gas Law
The Combined Gas Law can be further extended to incorporate the number of moles of gas, resulting in the Ideal Gas Law.
The Ideal Gas Law: PV = nRT
Where:
– P is pressure
– V is volume
– n is the number of moles
– R is the ideal gas constant
– T is temperature
The Ideal Gas Law provides a more comprehensive description of gas behavior, taking into account the amount of gas present. It is a powerful tool for predicting and understanding the behavior of gases in various applications.
Final Wrap-Up
Boyle’s Law is a cornerstone of our understanding of gases. It explains how gases behave under different conditions, and it has numerous applications in various fields. From the way we breathe to the operation of hydraulic systems, Boyle’s Law plays a vital role in our daily lives. Its simplicity and elegance make it a fundamental concept in science, and its applications continue to shape our world in profound ways.
FAQ Explained
What is the significance of Boyle’s Law?
Boyle’s Law is significant because it helps us understand the behavior of gases and predict how they will react under different conditions. This knowledge is crucial in various fields, such as medicine, engineering, and everyday life.
Can Boyle’s Law be applied to all gases?
Boyle’s Law is most accurate for ideal gases, which are theoretical gases that follow certain assumptions. Real gases may deviate from ideal behavior, especially at high pressures and low temperatures.
How does Boyle’s Law relate to breathing?
Our lungs work based on Boyle’s Law. When we inhale, our diaphragm contracts, increasing the volume of our chest cavity. This decrease in pressure causes air to flow into our lungs. When we exhale, the diaphragm relaxes, decreasing the volume of our chest cavity and increasing the pressure, forcing air out.
What are some examples of Boyle’s Law in everyday life?
Boyle’s Law is evident in various everyday scenarios, such as:
- Pumping air into a bicycle tire
- Inflating a balloon
- Using a syringe to draw liquid
- The operation of a scuba diving tank