What is the first law in thermodynamics – What is the First Law of Thermodynamics? This fundamental principle of physics governs the flow of energy in our universe, laying the foundation for understanding how engines work, how chemical reactions occur, and even how life itself sustains itself. It’s a law that governs the very fabric of our existence, shaping everything from the smallest atom to the largest star.
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another. This principle is central to understanding energy transformations, from the burning of fuel to the growth of plants, and it has profound implications for our understanding of the universe and its limitations.
Introduction to Thermodynamics

Thermodynamics is a fundamental branch of physics that deals with heat, work, temperature, and their relation to energy, radiation, and physical properties of matter. It is a cornerstone of science and engineering, providing a framework for understanding energy transformations and their impact on various systems. From the operation of engines and power plants to the behavior of chemical reactions and biological processes, thermodynamics plays a crucial role in explaining and predicting the behavior of the physical world around us.
Energy and Its Forms
Energy is a fundamental concept in thermodynamics, representing the capacity to do work. It exists in various forms, each possessing unique characteristics and potential for transformation. The primary forms of energy include:
- Kinetic Energy: The energy possessed by an object due to its motion. It is directly proportional to the object’s mass and the square of its velocity. For example, a moving car possesses kinetic energy.
- Potential Energy: The energy stored in an object due to its position or configuration. For instance, a book held above the ground possesses potential energy due to its height.
- Thermal Energy: The energy associated with the random motion of atoms and molecules within a system. It is directly proportional to the temperature of the system. For example, a hot cup of coffee has higher thermal energy than a cold glass of water.
- Chemical Energy: The energy stored within the bonds of molecules. When chemical bonds are broken or formed, energy is released or absorbed. For example, the combustion of fuels releases chemical energy.
- Nuclear Energy: The energy stored within the nucleus of an atom. Nuclear reactions, such as fission and fusion, release enormous amounts of energy. For example, nuclear power plants generate electricity by harnessing nuclear energy.
- Electromagnetic Energy: The energy associated with electric and magnetic fields. It is carried by photons, which are massless particles that travel at the speed of light. For example, sunlight is a form of electromagnetic energy.
Importance of Energy Transformations
Understanding energy transformations is crucial in various fields, including:
- Engineering: Thermodynamics provides the foundation for designing and optimizing engines, power plants, and other energy-related systems. It helps engineers to determine the efficiency of energy conversion processes and to minimize energy losses.
- Chemistry: Thermodynamics is essential for understanding chemical reactions and predicting their feasibility and equilibrium conditions. It allows chemists to determine the energy changes involved in chemical processes and to develop new synthetic pathways.
- Biology: Thermodynamics plays a critical role in understanding the energy flow within living organisms. It helps biologists to study metabolic processes, energy storage, and the mechanisms of biological work.
- Environmental Science: Thermodynamics is crucial for analyzing energy flows in ecosystems and understanding the environmental impact of human activities. It helps environmental scientists to assess the efficiency of energy utilization and to develop sustainable energy solutions.
The First Law of Thermodynamics
The First Law of Thermodynamics is a fundamental principle in physics that governs the relationship between heat, work, and internal energy. It is a statement of the conservation of energy, which asserts that energy cannot be created or destroyed, only transferred or transformed from one form to another. This law has profound implications for understanding various physical phenomena, from the operation of engines to the behavior of chemical reactions.
Forms of the First Law of Thermodynamics
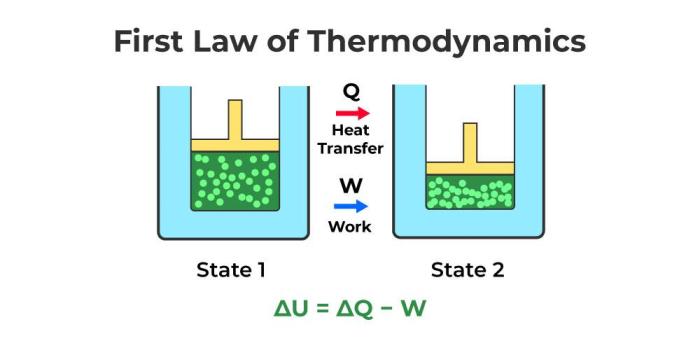
The First Law of Thermodynamics can be expressed in several equivalent forms, each emphasizing a different aspect of the relationship between heat, work, and internal energy.
The most common form is:
ΔU = Q – W
Where:
* ΔU represents the change in internal energy of a system.
* Q represents the heat added to the system.
* W represents the work done by the system.
This equation states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Another way to express the First Law is:
The total energy of an isolated system remains constant.
This statement emphasizes that energy is conserved within a closed system.
Relationship Between Heat, Work, and Internal Energy
The First Law of Thermodynamics establishes a clear relationship between heat, work, and internal energy.
* Heat is the transfer of thermal energy between objects at different temperatures.
* Work is the transfer of energy by means of a force acting over a distance.
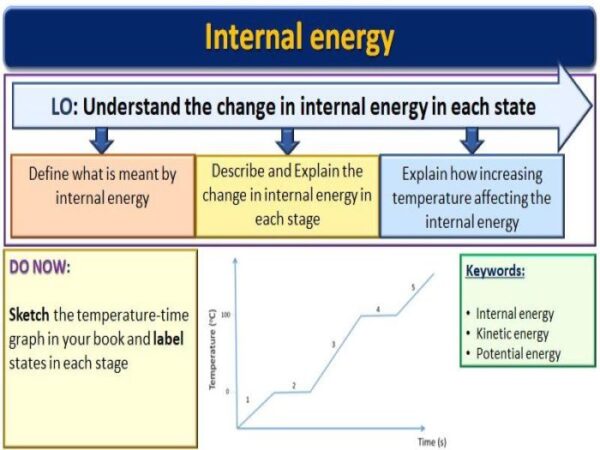
* Internal energy is the total energy of the molecules within a system, including kinetic energy (energy of motion) and potential energy (energy of position).
When heat is added to a system, its internal energy increases. Conversely, when work is done by a system, its internal energy decreases.
Examples of the First Law in Everyday Phenomena
The First Law of Thermodynamics is evident in many everyday phenomena:
* Heating a pot of water: When you heat a pot of water on a stove, you are adding heat to the water. This heat increases the internal energy of the water molecules, causing them to move faster and increasing the temperature of the water.
* A car engine: In a car engine, the combustion of fuel releases heat, which is used to expand gases and drive pistons. This work done by the expanding gases is then used to power the car.
* A refrigerator: A refrigerator works by transferring heat from the inside of the refrigerator to the outside. This process requires work to be done by the refrigerator’s compressor.
Applications of the First Law
The First Law of Thermodynamics, which states that energy cannot be created or destroyed but only transformed from one form to another, has profound implications across various fields. Its application extends beyond theoretical understanding to practical applications in diverse areas, influencing the design and optimization of systems and processes.
Engineering
The First Law is fundamental in engineering, particularly in the design and analysis of systems that involve energy transfer and transformation. It is crucial for understanding and predicting the efficiency of various engineering systems, including engines, power plants, and refrigeration systems.
- Engines: The First Law is applied to determine the efficiency of internal combustion engines, which convert chemical energy from fuel into mechanical energy. The efficiency of an engine is calculated by dividing the work output by the heat input. By understanding the First Law, engineers can optimize engine design to maximize efficiency and minimize energy losses.
- Power Plants: Power plants generate electricity by converting heat energy into mechanical energy, which then drives generators. The First Law helps engineers design power plants to maximize energy conversion efficiency. It also guides the selection of appropriate fuels and the optimization of combustion processes.
- Refrigeration Systems: Refrigeration systems use the First Law to transfer heat from a cold environment to a warmer environment. The efficiency of a refrigeration system is measured by its coefficient of performance (COP), which represents the ratio of heat removed to work input. The First Law is used to analyze the energy balance in refrigeration systems and to improve their efficiency.
Chemistry
The First Law of Thermodynamics plays a crucial role in understanding chemical reactions and their associated energy changes. It provides a framework for analyzing the energy balance in chemical processes and predicting the feasibility of reactions.
- Thermochemistry: The First Law forms the basis of thermochemistry, which studies the heat changes associated with chemical reactions. It is used to determine the enthalpy change (ΔH) of a reaction, which represents the heat absorbed or released during the reaction. The First Law also helps in understanding the relationship between enthalpy change, internal energy change, and work done in a chemical reaction.
- Chemical Equilibrium: The First Law is essential for understanding chemical equilibrium, which describes the state where the rates of forward and reverse reactions are equal. The First Law helps in determining the equilibrium constant (K), which indicates the relative amounts of reactants and products at equilibrium. By applying the First Law, chemists can predict the direction of a reaction and the extent to which it will proceed.
Biology
The First Law is fundamental to understanding energy flow in living organisms. It explains how energy is transferred and transformed within biological systems, supporting life processes such as growth, reproduction, and movement.
- Metabolism: The First Law governs metabolic processes, which involve the breakdown of nutrients to release energy and the synthesis of complex molecules. The First Law helps biologists understand the energy balance in metabolic reactions, including the efficiency of energy conversion and the energy requirements for different biological processes.
- Photosynthesis: Photosynthesis is the process by which plants convert light energy into chemical energy stored in glucose. The First Law helps explain the energy conversion efficiency of photosynthesis and the factors that influence the rate of energy capture.
Implications of the First Law
The First Law of Thermodynamics, with its fundamental principle of energy conservation, has profound implications that extend far beyond the realm of traditional thermodynamics. It serves as a cornerstone for understanding energy interactions in various fields, shaping our approach to energy conservation, technological advancements, and even our perception of the universe.
Energy Conservation and Sustainability
The First Law emphasizes that energy cannot be created or destroyed, only transformed from one form to another. This principle is central to our understanding of energy conservation and sustainability. By recognizing that energy is finite and cannot be replenished, we are compelled to utilize it efficiently and responsibly. The First Law provides a framework for designing energy systems that minimize energy waste and maximize efficiency, leading to reduced environmental impact and sustainable energy practices. For example, renewable energy technologies, such as solar and wind power, rely on the First Law to harness and convert naturally occurring energy sources into usable forms.
Development of New Energy Technologies, What is the first law in thermodynamics
The First Law is a guiding principle in the development of new energy technologies. By understanding the limits and possibilities of energy conversion, scientists and engineers can design systems that efficiently transform energy from one form to another. For instance, the development of fuel cells, which convert chemical energy into electrical energy, is driven by the First Law. Similarly, the advancement of energy storage technologies, such as batteries and capacitors, relies on the principles of energy conservation and transformation Artikeld by the First Law.
Understanding the Limits of Energy Conversion
The First Law also reveals the inherent limitations of energy conversion. It states that while energy can be transformed, it cannot be created or destroyed. This implies that no energy conversion process is 100% efficient, as some energy is inevitably lost as heat or other forms of unusable energy. Understanding these limitations allows us to optimize energy conversion processes and minimize energy waste. For example, in power plants, the First Law helps us understand the theoretical limits of energy conversion efficiency and guides the design of systems that minimize energy losses.
Ultimate Conclusion: What Is The First Law In Thermodynamics
The First Law of Thermodynamics is a cornerstone of our understanding of the universe. It dictates the flow of energy, from the smallest particles to the largest galaxies, and has profound implications for our understanding of the universe’s limits and possibilities. It guides our development of energy technologies, our efforts to conserve energy, and our exploration of the universe’s fundamental principles. The First Law of Thermodynamics is not just a scientific principle, but a guiding force for our understanding of the world around us.
Top FAQs
What are some everyday examples of the First Law of Thermodynamics?
Heating a pot of water on a stove is a great example. The heat from the stove is transferred to the pot, increasing its internal energy and causing the water to boil. The energy is not created, but simply transformed from heat energy to thermal energy of the water.
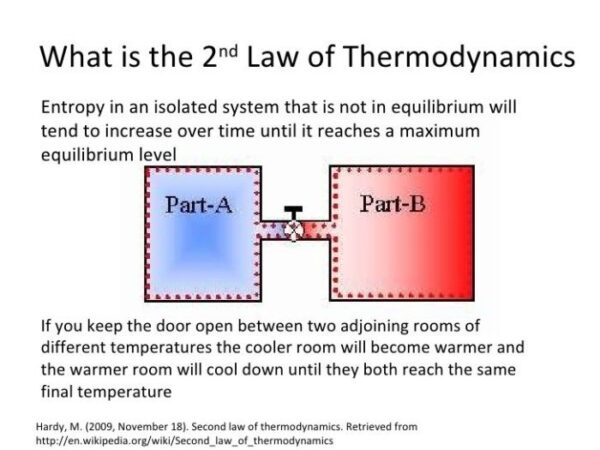
How does the First Law relate to the concept of entropy?
While the First Law states that energy is conserved, it doesn’t say anything about the quality of energy. The Second Law of Thermodynamics introduces the concept of entropy, which states that the total entropy of an isolated system can never decrease over time. This means that energy transformations always result in some energy being lost as unusable heat, leading to a decrease in the system’s ability to do work.
Can the First Law of Thermodynamics be violated?
No, the First Law of Thermodynamics is a fundamental law of physics, and there is no known evidence to suggest that it can be violated. However, it is important to note that the law applies to closed systems, where there is no exchange of energy with the surroundings. In open systems, energy can be exchanged, and the First Law applies to the total energy of the system and its surroundings.