A balanced chemical reaction obeys the law of responses – Balanced chemical reactions: obeying the law of conservation of mass, is a fundamental principle in chemistry. This law states that in a closed system, the total mass of the reactants before a chemical reaction must equal the total mass of the products after the reaction. This principle ensures that matter is neither created nor destroyed during a chemical transformation. It’s a cornerstone of our understanding of how chemicals interact and change, providing a framework for predicting the outcome of reactions and calculating the amounts of substances involved.
Imagine a chemical reaction as a recipe. Just as a recipe requires specific ingredients in precise amounts to produce a desired dish, a balanced chemical equation ensures that the atoms involved in the reaction are accounted for on both sides of the equation. This balance reflects the fundamental law of conservation of mass, ensuring that no atoms are lost or gained during the chemical transformation. Understanding this principle is essential for comprehending the intricate world of chemistry and its applications in various fields, from pharmaceuticals to environmental science.
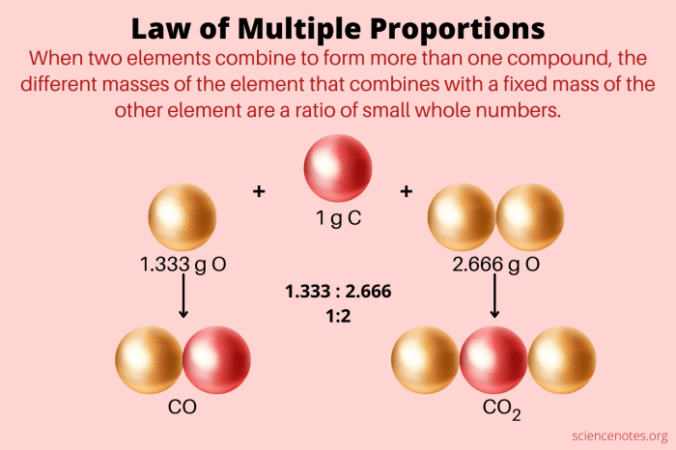
The Law of Conservation of Mass
The Law of Conservation of Mass is a fundamental principle in chemistry that states that matter cannot be created or destroyed in ordinary chemical reactions. This means that the total mass of the reactants (the substances that react) must equal the total mass of the products (the substances formed in the reaction).
Demonstration of the Law in Balanced Chemical Equations
Balanced chemical equations are a visual representation of the Law of Conservation of Mass. They demonstrate the equal number of atoms of each element on both sides of the equation.
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 → 2H2O
In this equation, there are four hydrogen atoms (2 x 2) and two oxygen atoms on both the reactant and product sides. This ensures that the total mass of the reactants (hydrogen and oxygen) is equal to the total mass of the product (water).
Significance of the Law of Conservation of Mass, A balanced chemical reaction obeys the law of responses
The Law of Conservation of Mass is significant because it allows us to predict the amount of products that will be formed in a chemical reaction, given the amount of reactants. This is crucial for many applications, such as:
- Stoichiometry: Calculating the quantities of reactants and products involved in chemical reactions.
- Industrial Chemistry: Optimizing chemical processes and ensuring efficient use of resources.
- Environmental Chemistry: Understanding the fate of pollutants and the impact of chemical reactions on the environment.
Balancing Chemical Equations
Chemical equations are a shorthand way of representing chemical reactions. They show the reactants (the substances that react) and the products (the substances that are formed) of a reaction. However, to accurately represent the reaction and ensure the law of conservation of mass is followed, chemical equations need to be balanced.
Balancing Chemical Equations
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element on the reactant side of the equation equals the number of atoms of that element on the product side.
Here are the steps involved in balancing a chemical equation:
- Write the unbalanced chemical equation. This is the starting point for the balancing process.
- Count the number of atoms of each element on both sides of the equation. This helps identify which elements need to be balanced.
- Adjust the coefficients in front of the chemical formulas to balance the number of atoms of each element. It is important to note that only coefficients can be changed; subscripts in the chemical formulas should not be altered.
- Check that the number of atoms of each element is the same on both sides of the equation. If the equation is balanced, the number of atoms of each element on the reactants side will equal the number of atoms of that element on the products side.
Balanced vs. Unbalanced Chemical Equations
A balanced chemical equation represents a chemical reaction accurately, as it follows the law of conservation of mass. It shows that the total mass of the reactants equals the total mass of the products. In contrast, an unbalanced chemical equation does not represent the reaction accurately, as it does not follow the law of conservation of mass.
For example, consider the reaction between hydrogen and oxygen to form water. The unbalanced equation is:
H2 + O2 → H2O
This equation is unbalanced because there are two oxygen atoms on the reactants side and only one on the products side. To balance the equation, we need to adjust the coefficients:
2H2 + O2 → 2H2O
Now the equation is balanced, as there are four hydrogen atoms and two oxygen atoms on both sides of the equation. This balanced equation accurately represents the reaction and shows that the total mass of the reactants (two molecules of hydrogen and one molecule of oxygen) equals the total mass of the products (two molecules of water).
Stoichiometry and Balanced Reactions
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It provides a framework for understanding how much of each substance is involved in a chemical reaction and how much product can be formed. Balanced chemical equations are essential for performing stoichiometric calculations, as they represent the exact proportions of reactants and products involved in a reaction.
Relationship between Stoichiometry and Balanced Chemical Equations
Balanced chemical equations are the foundation of stoichiometry. They provide the mole ratios between reactants and products, which are crucial for calculating the amounts of substances involved in a reaction.
The coefficients in a balanced chemical equation represent the number of moles of each reactant and product involved in the reaction.
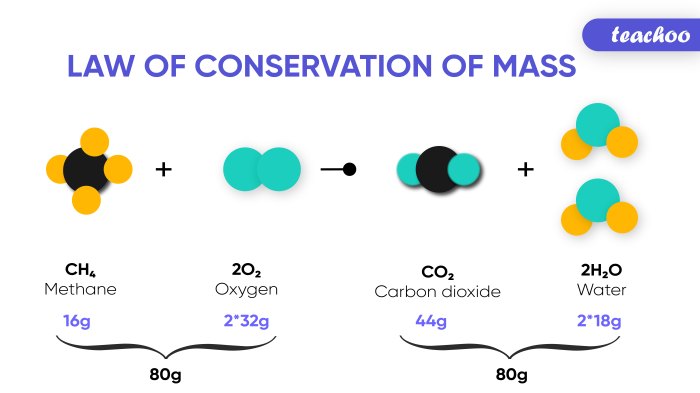
For example, the balanced chemical equation for the combustion of methane is:
CH4 + 2O2 → CO2 + 2H2O
This equation indicates that one mole of methane (CH4) reacts with two moles of oxygen (O2) to produce one mole of carbon dioxide (CO2) and two moles of water (H2O). This information is essential for stoichiometric calculations.
Calculating the Amount of Reactants and Products
Balanced chemical equations are used to calculate the amount of reactants and products involved in a chemical reaction using the following steps:
- Write the balanced chemical equation for the reaction.
- Convert the given mass or volume of a reactant or product to moles. This can be done using the molar mass of the substance. For example, to convert 10 grams of sodium chloride (NaCl) to moles, you would divide the mass by the molar mass of NaCl (58.44 g/mol). This would give you 0.171 moles of NaCl.
- Use the mole ratios from the balanced chemical equation to determine the number of moles of other reactants or products involved in the reaction. For example, if the balanced equation shows that 2 moles of NaCl react with 1 mole of silver nitrate (AgNO3), and you have 0.171 moles of NaCl, then you would have 0.0855 moles of AgNO3.
- Convert the number of moles of the desired substance back to grams or volume, if necessary.
Real-World Applications of Stoichiometry
Stoichiometry has numerous applications in various fields, including:
- Industrial Chemistry: Stoichiometry is used to determine the optimal amounts of reactants to use in industrial processes to maximize product yield and minimize waste. For example, in the production of ammonia (NH3) using the Haber-Bosch process, stoichiometry is used to calculate the exact amounts of nitrogen (N2) and hydrogen (H2) needed to produce the desired amount of ammonia.
- Pharmaceutical Industry: Stoichiometry is crucial in the pharmaceutical industry for determining the precise amounts of ingredients needed to produce drugs and medications. This ensures the correct dosage and effectiveness of the medication.
- Environmental Science: Stoichiometry is used to assess the impact of pollutants on the environment. For example, it can be used to calculate the amount of carbon dioxide (CO2) released from the combustion of fossil fuels.
Types of Chemical Reactions
Chemical reactions are fundamental processes that involve the rearrangement of atoms and molecules, leading to the formation of new substances. Understanding the different types of chemical reactions is crucial for comprehending the diverse chemical phenomena that occur in our world.
Classifying Chemical Reactions
Chemical reactions can be classified into various types based on the changes in the reactants and products. The following are some of the most common types of chemical reactions:
- Combination Reaction: A combination reaction involves the formation of a single product from two or more reactants. These reactions are also known as synthesis reactions.
A + B → AB
For example, the reaction of sodium (Na) with chlorine (Cl2) to form sodium chloride (NaCl) is a combination reaction:
2Na + Cl2 → 2NaCl
- Decomposition Reaction: A decomposition reaction is the opposite of a combination reaction. It involves the breakdown of a single reactant into two or more products.
AB → A + B
For example, the decomposition of calcium carbonate (CaCO3) into calcium oxide (CaO) and carbon dioxide (CO2) is a decomposition reaction:
CaCO3 → CaO + CO2
- Single Replacement Reaction: A single replacement reaction involves the displacement of one element from a compound by another element.
A + BC → AC + B
For example, the reaction of zinc (Zn) with hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas (H2) is a single replacement reaction:
Zn + 2HCl → ZnCl2 + H2
- Double Replacement Reaction: A double replacement reaction involves the exchange of ions between two reactants, resulting in the formation of two new compounds.
AB + CD → AD + CB
For example, the reaction of silver nitrate (AgNO3) with sodium chloride (NaCl) to form silver chloride (AgCl) and sodium nitrate (NaNO3) is a double replacement reaction:
AgNO3 + NaCl → AgCl + NaNO3
- Combustion Reaction: A combustion reaction involves the rapid reaction between a substance with an oxidant, usually oxygen, to produce heat and light.
Fuel + O2 → CO2 + H2O + Heat + Light
For example, the burning of methane (CH4) in air is a combustion reaction:
CH4 + 2O2 → CO2 + 2H2O
The Law of Conservation of Mass in Chemical Reactions
The law of conservation of mass states that in a closed system, the total mass of the reactants before a chemical reaction must equal the total mass of the products after the reaction. This law is fundamental to understanding chemical reactions and ensures that matter is not created or destroyed during a chemical process.
| Type of Reaction | Balanced Equation Format | Description of the Law of Conservation of Mass |
|---|---|---|
| Combination Reaction | A + B → AB | The total mass of reactants A and B is equal to the mass of the product AB. |
| Decomposition Reaction | AB → A + B | The total mass of reactant AB is equal to the combined mass of products A and B. |
| Single Replacement Reaction | A + BC → AC + B | The total mass of reactants A and BC is equal to the combined mass of products AC and B. |
| Double Replacement Reaction | AB + CD → AD + CB | The total mass of reactants AB and CD is equal to the combined mass of products AD and CB. |
| Combustion Reaction | Fuel + O2 → CO2 + H2O + Heat + Light | The total mass of fuel and oxygen is equal to the combined mass of carbon dioxide, water, and any other products, even though some of the mass is released as heat and light. |
The Importance of Balanced Reactions
Balancing chemical equations is fundamental to understanding chemical reactions and their implications. It ensures that the law of conservation of mass is upheld, which states that matter cannot be created or destroyed in ordinary chemical and physical changes.
Consequences of Unbalanced Equations
Unbalanced chemical equations can lead to significant errors in scientific calculations and predictions. They fail to represent the accurate stoichiometry of a reaction, which is the quantitative relationship between reactants and products. This can result in incorrect estimations of:
- The amount of reactants needed to produce a specific amount of product.
- The amount of product that can be obtained from a given amount of reactant.
- The energy changes involved in a reaction.
For example, consider the incomplete combustion of methane, a reaction that produces carbon monoxide, a toxic gas. An unbalanced equation might appear as:
CH4 + O2 → CO + H2O
This equation does not reflect the actual stoichiometry of the reaction. The balanced equation is:
2CH4 + 3O2 → 2CO + 4H2O
Using the unbalanced equation, one might incorrectly calculate the amount of oxygen needed for combustion, leading to insufficient oxygen and the production of harmful carbon monoxide.
Applications of Balanced Reactions
Balanced chemical equations are indispensable in various fields, including:
- Chemistry: Balancing equations helps chemists understand the mechanisms of reactions, predict product yields, and optimize reaction conditions. For example, in the synthesis of new materials, balancing equations helps determine the precise amounts of reactants needed to obtain the desired product.
- Environmental Science: Balanced equations are crucial for understanding and mitigating environmental pollution. For instance, understanding the balanced equation for the combustion of fossil fuels helps assess the amount of greenhouse gases released into the atmosphere.
- Engineering: In chemical engineering, balanced equations are essential for designing and optimizing chemical processes, such as the production of fertilizers, pharmaceuticals, and plastics. For example, balancing equations for reactions involved in the production of fertilizers helps ensure efficient use of raw materials and minimize waste generation.
Closure
In conclusion, the concept of balanced chemical reactions and the law of conservation of mass is essential to understanding chemical reactions. By ensuring that the number of atoms of each element is equal on both sides of a chemical equation, we uphold this fundamental principle, allowing us to accurately predict the outcome of reactions and calculate the amounts of reactants and products involved. This knowledge empowers us to explore the intricate world of chemistry and its profound impact on our lives, from the creation of life-saving medications to the development of sustainable energy solutions.
Question Bank: A Balanced Chemical Reaction Obeys The Law Of Responses
What are the practical implications of balancing chemical equations?
Balancing chemical equations is crucial for accurate predictions of reaction outcomes and calculations of reactant and product amounts. This is essential in various applications, including industrial processes, environmental monitoring, and laboratory experiments.
How do balanced equations relate to the real world?
Balanced equations are used in many real-world scenarios, such as determining the amount of reactants needed for a specific chemical reaction in industrial production, predicting the amount of pollutants released from a chemical process, and designing efficient combustion reactions for power generation.
Can a chemical reaction occur without obeying the law of conservation of mass?
No, a chemical reaction cannot occur without obeying the law of conservation of mass. This law is a fundamental principle of chemistry, and any reaction that appears to violate it is likely to be incomplete or involve an error in measurement.