What is beers law – What is Beer-Lambert Law? This fundamental principle in chemistry unlocks the secrets of how light interacts with matter, revealing the concentration of a substance within a solution. It’s a powerful tool that finds its applications in various fields, from analytical chemistry to biochemistry and even environmental science.
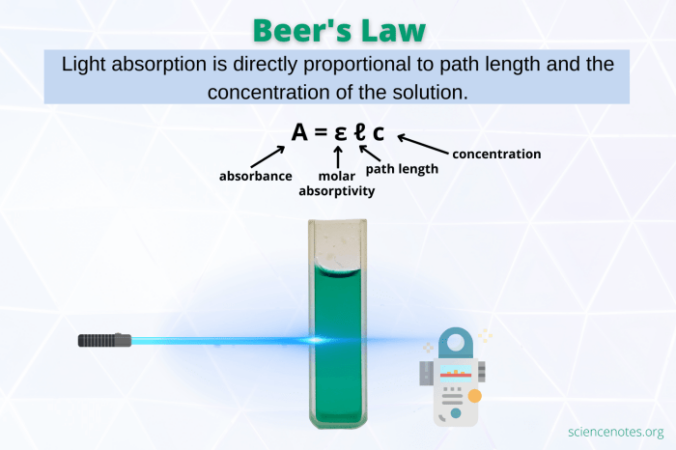
The Beer-Lambert Law establishes a direct relationship between the absorbance of light by a solution and the concentration of the substance dissolved in it. This relationship is also influenced by the path length, which is the distance the light travels through the solution. This means that the more concentrated the solution, the more light it will absorb. Similarly, the longer the path length, the greater the absorbance.
Introduction to Beer-Lambert Law
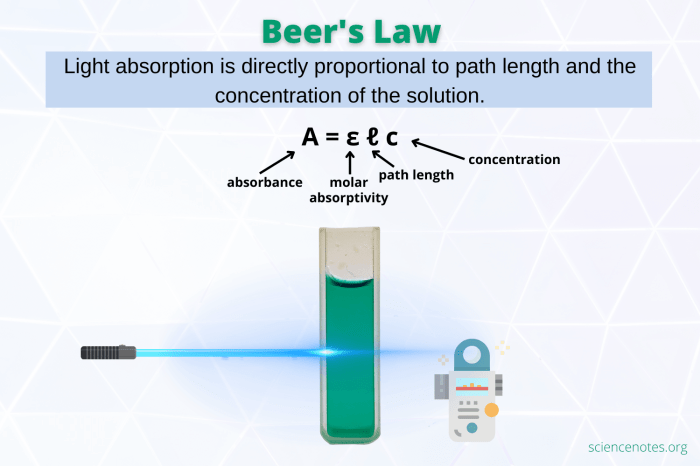
The Beer-Lambert Law, also known as Beer’s Law, is a fundamental principle in chemistry that describes the relationship between the absorbance of light by a solution and the concentration of the absorbing species in the solution. This law is widely used in analytical chemistry for quantitative analysis, where it enables scientists to determine the concentration of an unknown substance by measuring the amount of light it absorbs.
The Beer-Lambert Law states that the absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of the light beam through the solution.
Relationship between Absorbance, Concentration, and Path Length
The Beer-Lambert Law can be expressed mathematically as:
A = εbc
Where:
* A is the absorbance
* ε is the molar absorptivity, a constant that is specific to the absorbing species and the wavelength of light used.
* b is the path length, the distance that the light travels through the solution.
* c is the concentration of the absorbing species.
This equation reveals that absorbance is directly proportional to both concentration and path length. If the concentration of the absorbing species is doubled, the absorbance will also double, assuming the path length remains constant. Similarly, if the path length is doubled, the absorbance will also double, assuming the concentration remains constant.
Applications of Beer-Lambert Law
The Beer-Lambert Law has numerous applications in various fields, including:
* Analytical Chemistry: It is widely used for quantitative analysis, such as determining the concentration of a substance in a solution.
* Spectrophotometry: The Beer-Lambert Law is the foundation of spectrophotometry, a technique used to measure the absorbance of light by a sample at different wavelengths.
* Environmental Monitoring: The Beer-Lambert Law is used to measure the concentration of pollutants in air, water, and soil.
* Medical Diagnostics: The Beer-Lambert Law is used in medical diagnostics to measure the concentration of various substances in blood and urine, such as glucose, proteins, and enzymes.
* Food Science: The Beer-Lambert Law is used to measure the concentration of pigments in food, such as chlorophyll in vegetables and anthocyanins in fruits.
Mathematical Expression of Beer-Lambert Law
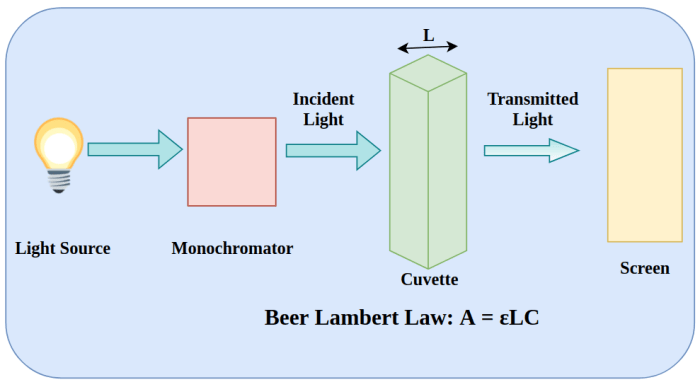
The Beer-Lambert Law is a fundamental principle in spectrophotometry, a technique used to measure the absorbance of light by a solution. It establishes a direct relationship between the absorbance of a solution, the concentration of the analyte, and the path length of the light beam through the solution.
The Beer-Lambert Law can be mathematically expressed as:
A = εbc
Variables in the Beer-Lambert Law
The equation contains four key variables:
- A: Absorbance – This is the amount of light absorbed by the solution. It is measured as a dimensionless quantity, typically expressed as a decimal value.
- ε: Molar absorptivity – This is a constant that represents the ability of a substance to absorb light at a specific wavelength. It is measured in units of L mol-1 cm-1. Each substance has a unique molar absorptivity at a given wavelength, making it a useful parameter for identifying and quantifying substances.
- b: Path length – This is the distance the light beam travels through the solution. It is typically measured in centimeters (cm).
- c: Concentration – This is the amount of analyte present in the solution. It is typically measured in units of moles per liter (mol/L) or molarity (M).
Calculating Absorbance
The Beer-Lambert Law can be used to calculate the absorbance of a solution if the molar absorptivity, concentration, and path length are known. For example, if a solution has a molar absorptivity of 1000 L mol-1 cm-1, a concentration of 0.1 mol/L, and a path length of 1 cm, the absorbance can be calculated as follows:
A = εbc = (1000 L mol-1 cm-1)(0.1 mol/L)(1 cm) = 100
Therefore, the absorbance of the solution would be 100.
Factors Affecting Absorbance
Absorbance, a key parameter in Beer-Lambert Law, is not a fixed value but can be influenced by several factors. Understanding these factors is crucial for accurate absorbance measurements and the reliable application of Beer-Lambert Law in various analytical techniques.
Concentration
The concentration of the analyte is directly proportional to absorbance. As the concentration of the analyte increases, more light is absorbed, resulting in a higher absorbance value. This relationship is fundamental to Beer-Lambert Law, which states that absorbance is directly proportional to the concentration of the absorbing species.
Absorbance (A) = εbc
where:
* ε is the molar absorptivity, a constant specific to the analyte and wavelength.
* b is the path length, the distance the light travels through the sample.
* c is the concentration of the analyte.
Path Length
The path length, or the distance the light beam travels through the sample, also affects absorbance. As the path length increases, more of the light beam interacts with the analyte molecules, leading to greater absorption and a higher absorbance value. This is consistent with Beer-Lambert Law, which states that absorbance is directly proportional to the path length.
Wavelength
The wavelength of light used for measurement significantly impacts absorbance. Each analyte exhibits a unique absorption spectrum, with maximum absorbance occurring at a specific wavelength known as the wavelength of maximum absorbance (λmax). The molar absorptivity (ε) is highest at λmax, resulting in the greatest sensitivity for detecting the analyte at this wavelength.
Molar Absorptivity
Molar absorptivity (ε) is a constant that represents the ability of a substance to absorb light at a specific wavelength. It is a characteristic property of the analyte and the wavelength used. Higher molar absorptivity indicates that the substance absorbs more light at that particular wavelength. Molar absorptivity plays a crucial role in determining absorbance, as it directly relates the absorbance to the concentration and path length.
Deviations from Beer-Lambert Law
While Beer-Lambert Law provides a fundamental framework for understanding the relationship between absorbance, concentration, and path length, deviations from this law can occur under certain conditions. These deviations can arise from factors such as:
- High Concentration: At high concentrations, the analyte molecules can interact with each other, leading to changes in the absorption characteristics and deviations from linearity.
- Chemical Reactions: If the analyte undergoes chemical reactions in the solution, it can affect its absorbance and lead to deviations from Beer-Lambert Law.
- Scattering: If the solution contains particles that scatter light, it can interfere with the absorbance measurement and lead to deviations.
- Non-ideal Behavior: Deviations can also occur due to non-ideal behavior of the analyte, such as association or dissociation in solution.
Deviations from Beer-Lambert Law can lead to inaccurate absorbance measurements and affect the reliability of analytical results. It is important to be aware of these potential deviations and to take appropriate steps to minimize their impact.
Applications of Beer-Lambert Law
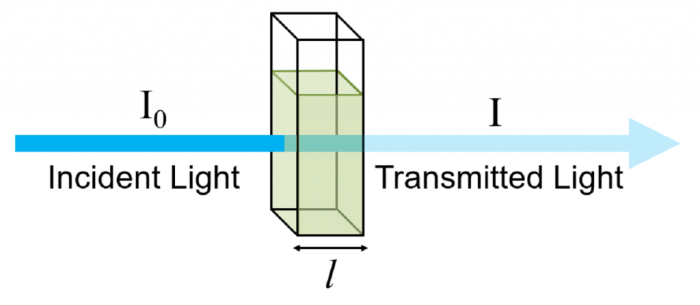
The Beer-Lambert Law, a fundamental principle in spectroscopy, finds widespread applications in various scientific disciplines. This law establishes a direct relationship between the absorbance of a solution and the concentration of the analyte, providing a powerful tool for quantitative analysis.
Analytical Chemistry
The Beer-Lambert Law is extensively employed in analytical chemistry for determining the concentration of substances in solutions. This technique, known as spectrophotometry, involves measuring the absorbance of a solution at a specific wavelength and utilizing the Beer-Lambert Law to calculate the concentration.
- Quantitative Analysis of Pharmaceutical Compounds: The Beer-Lambert Law is instrumental in determining the concentration of active pharmaceutical ingredients in drug formulations. Spectrophotometric analysis using this law ensures accurate dosage and quality control in pharmaceutical manufacturing.
- Food Chemistry: The Beer-Lambert Law is applied in food analysis to quantify various components, such as pigments, preservatives, and vitamins. For instance, measuring the absorbance of a solution at a specific wavelength allows the determination of the concentration of vitamin C in fruit juices.
- Environmental Monitoring: The Beer-Lambert Law is used to monitor the levels of pollutants in water and air. For example, measuring the absorbance of a water sample at a specific wavelength can determine the concentration of heavy metals like lead or mercury.
Biochemistry
In biochemistry, the Beer-Lambert Law plays a crucial role in studying the properties of biological molecules.
- Protein Quantification: Spectrophotometry based on the Beer-Lambert Law is widely used to determine the concentration of proteins in solutions. This technique is essential for studying protein function, enzyme kinetics, and protein-protein interactions.
- DNA and RNA Analysis: The Beer-Lambert Law is used to quantify DNA and RNA in various applications, including gene expression analysis, molecular diagnostics, and forensic science.
- Enzyme Activity Assays: Spectrophotometric assays utilizing the Beer-Lambert Law are employed to measure the activity of enzymes by monitoring the rate of product formation or substrate consumption.
Environmental Science
The Beer-Lambert Law finds applications in environmental science for monitoring and analyzing various environmental parameters.
- Water Quality Analysis: The Beer-Lambert Law is used to determine the concentration of dissolved substances in water, including nutrients, pollutants, and contaminants. This information is crucial for assessing water quality and ensuring public health.
- Air Quality Monitoring: Spectrophotometric methods based on the Beer-Lambert Law are used to measure the concentration of air pollutants, such as ozone, nitrogen dioxide, and sulfur dioxide. This data is essential for monitoring air quality and implementing pollution control measures.
- Soil Analysis: The Beer-Lambert Law can be applied to analyze the composition of soil, including the determination of organic matter content, nutrient levels, and the presence of heavy metals.
Instrumentation and Techniques: What Is Beers Law
Spectrophotometry is a powerful analytical technique that utilizes the interaction of light with matter to determine the concentration of a substance. It plays a crucial role in implementing Beer-Lambert Law, enabling us to measure the absorbance of a solution and relate it to the concentration of the analyte.
Spectrophotometry Principles
Spectrophotometry is based on the principle that each substance absorbs and transmits light at specific wavelengths. The process involves passing a beam of light through a sample and measuring the amount of light that passes through it. The ratio of the incident light intensity to the transmitted light intensity is known as the transmittance (T), while the negative logarithm of the transmittance is called the absorbance (A). The absorbance is directly proportional to the concentration of the analyte in the solution, as described by Beer-Lambert Law.
Types of Spectrophotometers, What is beers law
Spectrophotometers come in various types, each employing different working principles to measure absorbance. Here are some common types:
- Single-beam spectrophotometers: These spectrophotometers use a single light beam that passes through the sample and then to the detector. They are simple and cost-effective but require frequent calibration to account for variations in light source intensity.
- Double-beam spectrophotometers: These spectrophotometers split the light beam into two paths, one passing through the sample and the other through a reference solution. The detector measures the difference in light intensity between the two beams, providing a more accurate measurement of absorbance. They are more complex but offer greater accuracy and stability.
- UV-Vis spectrophotometers: These spectrophotometers operate in the ultraviolet (UV) and visible (Vis) regions of the electromagnetic spectrum. They are widely used in analytical chemistry, biochemistry, and pharmaceutical research for quantitative analysis, kinetic studies, and identification of compounds.
- Infrared (IR) spectrophotometers: These spectrophotometers operate in the infrared region of the electromagnetic spectrum. They are used to identify and quantify organic molecules based on their unique vibrational frequencies.
Calibration and Sample Preparation
Proper calibration and sample preparation are crucial for obtaining accurate and reliable absorbance measurements.
- Calibration: Calibration involves using a known standard solution of the analyte to establish a relationship between absorbance and concentration. A calibration curve is typically created by measuring the absorbance of solutions with known concentrations. This curve allows us to determine the concentration of an unknown sample by measuring its absorbance and interpolating on the curve.
- Sample Preparation: Sample preparation involves dissolving the analyte in a suitable solvent and adjusting the concentration to fall within the linear range of the Beer-Lambert Law. The sample should be free of any particulate matter or contaminants that could interfere with the absorbance measurement.
Beer-Lambert Law in Practice
The Beer-Lambert Law is a fundamental principle in analytical chemistry that describes the relationship between the absorbance of a solution and the concentration of the analyte. This law finds extensive applications in various fields, including environmental monitoring, pharmaceutical analysis, and clinical diagnostics. To understand the practical implications of this law, it is crucial to perform experiments that demonstrate its application.
A Simple Experiment Demonstrating the Beer-Lambert Law
This experiment involves the use of a spectrophotometer to measure the absorbance of solutions with varying concentrations of a colored analyte. The experiment aims to verify the linear relationship between absorbance and concentration as predicted by the Beer-Lambert Law.
Step-by-Step Procedure
- Prepare a series of solutions with known concentrations of the analyte. For example, you can use a stock solution of a colored dye, such as copper sulfate, and dilute it to create solutions with different concentrations.
- Using a spectrophotometer, measure the absorbance of each solution at a specific wavelength where the analyte absorbs maximally. This wavelength is typically determined by obtaining a spectrum of the analyte solution.
- Plot the absorbance values against the corresponding concentrations of the analyte. The resulting graph should be a straight line passing through the origin, indicating a direct proportionality between absorbance and concentration.
Analyzing the Experimental Data and Interpreting the Results
The slope of the linear graph obtained in step 3 represents the molar absorptivity (ε) of the analyte at the chosen wavelength. Molar absorptivity is a constant that is characteristic of a particular analyte and wavelength. The y-intercept of the graph should ideally be zero, confirming the Beer-Lambert Law’s prediction that absorbance is directly proportional to concentration.
The equation for the Beer-Lambert Law is:
A = εbc
where A is the absorbance, ε is the molar absorptivity, b is the path length of the light beam through the solution, and c is the concentration of the analyte.
Any deviations from linearity in the graph may indicate non-ideal conditions, such as the presence of interfering substances, exceeding the analyte’s concentration range, or errors in measurement.
Conclusion
Understanding Beer-Lambert Law opens doors to a world of scientific exploration. It allows us to quantify the concentration of substances, monitor chemical reactions, and analyze the composition of materials. From analyzing blood samples in medical labs to determining the concentration of pollutants in the environment, Beer-Lambert Law plays a crucial role in various scientific and technological advancements.
Clarifying Questions
What is the difference between absorbance and transmittance?
Absorbance is the amount of light absorbed by a solution, while transmittance is the amount of light that passes through the solution.
How can I determine the molar absorptivity of a substance?
Molar absorptivity can be determined experimentally by measuring the absorbance of a solution of known concentration at a specific wavelength and path length.
What are the limitations of Beer-Lambert Law?
Beer-Lambert Law is not always applicable, especially at high concentrations or when the solution exhibits non-ideal behavior.