What is Gay-Lussac’s Law in simple terms? It’s a fundamental principle in chemistry that explains the relationship between the temperature and volume of a gas. Discovered by the French chemist Gay-Lussac in the early 19th century, this law states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature. Imagine heating up a balloon – as the air inside gets hotter, it expands, making the balloon bigger! This simple concept has far-reaching implications in various fields, from weather forecasting to industrial processes.
Gay-Lussac’s Law is expressed mathematically as V₁/T₁ = V₂/T₂, where V represents volume and T represents temperature. This means that if you increase the temperature of a gas, its volume will also increase proportionally, and vice versa. The law holds true for ideal gases, which are theoretical gases that behave perfectly according to the gas laws. In real-world scenarios, however, gases may deviate slightly from this ideal behavior.
Applications of Gay-Lussac’s Law
Gay-Lussac’s Law, a fundamental principle in chemistry and physics, has numerous practical applications in various fields. It describes the direct relationship between the pressure and temperature of a gas, assuming a constant volume. This law is crucial in understanding and predicting the behavior of gases in diverse scenarios.
Weather Forecasting
Weather forecasting heavily relies on Gay-Lussac’s Law to predict changes in atmospheric pressure and temperature. Meteorologists use this law to understand how temperature variations affect air pressure and vice versa. For example, when air is heated, it expands and becomes less dense, leading to a decrease in pressure. Conversely, when air cools, it contracts and becomes denser, resulting in an increase in pressure. This knowledge helps meteorologists predict weather patterns, such as the formation of storms and changes in wind direction.
Hot Air Balloon Inflation
Hot air balloons are a prime example of Gay-Lussac’s Law in action. The principle behind their flight is based on the relationship between temperature and pressure. As hot air is injected into the balloon, it expands, creating a lower density compared to the surrounding cooler air. This density difference generates buoyancy, lifting the balloon into the air. The hotter the air inside the balloon, the greater the pressure difference, and the higher the balloon will rise.
Industrial Processes
Gay-Lussac’s Law finds wide application in various industrial processes. For instance, in chemical industries, it is crucial in controlling the pressure and temperature of reactions involving gases. By carefully monitoring and adjusting the temperature of a reaction vessel, engineers can ensure the desired pressure is maintained, optimizing the efficiency and safety of the process.
Limitations of Gay-Lussac’s Law
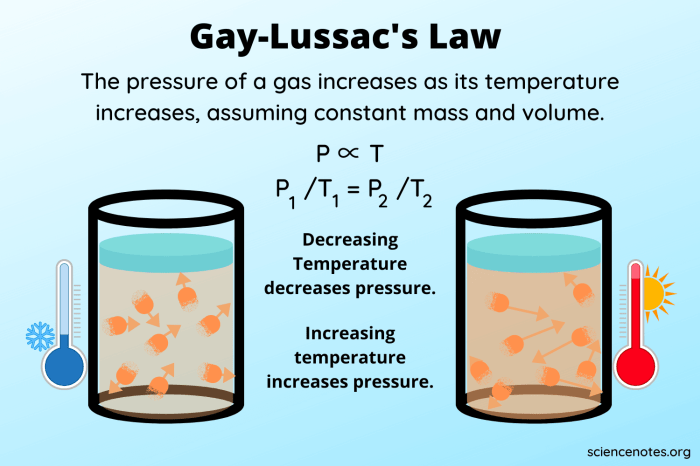
Gay-Lussac’s Law, like all gas laws, is based on ideal gas behavior. This means it holds true under specific conditions, and deviations can occur in real-world scenarios.
Gay-Lussac’s Law assumes that the gas behaves ideally, meaning that the gas molecules have negligible volume and do not interact with each other. These assumptions hold true for gases at low pressures and high temperatures. However, as pressure increases or temperature decreases, the gas molecules begin to interact more strongly, and their volume becomes more significant. This leads to deviations from Gay-Lussac’s Law.
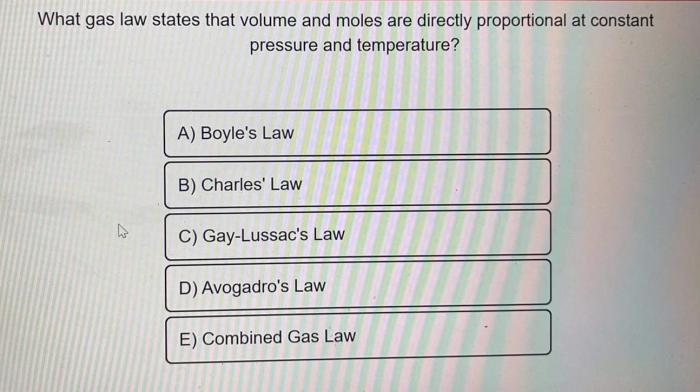
Comparison with Other Gas Laws, What is gay-lussac’s law in simple terms
Gay-Lussac’s Law is closely related to other gas laws, particularly Boyle’s Law and Charles’ Law. Understanding their similarities and differences helps clarify the limitations of Gay-Lussac’s Law.
- Boyle’s Law states that the volume of a gas is inversely proportional to its pressure at constant temperature. This means that as pressure increases, volume decreases, and vice versa. Gay-Lussac’s Law focuses on the relationship between temperature and pressure at constant volume, while Boyle’s Law explores the relationship between volume and pressure at constant temperature.
- Charles’ Law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. This means that as temperature increases, volume increases, and vice versa. Similar to Boyle’s Law, Charles’ Law explores the relationship between temperature and volume at constant pressure, whereas Gay-Lussac’s Law focuses on temperature and pressure at constant volume.
Gay-Lussac’s Law, Boyle’s Law, and Charles’ Law are all special cases of the Ideal Gas Law, which combines all three relationships into a single equation: PV = nRT.
Final Wrap-Up

Understanding Gay-Lussac’s Law allows us to predict how gases will behave under different temperature conditions. It’s a crucial tool for scientists, engineers, and even everyday people who want to understand the world around them. From predicting the expansion of hot air balloons to understanding how temperature affects the pressure in a car tire, Gay-Lussac’s Law provides a simple yet powerful framework for understanding the behavior of gases.
Quick FAQs: What Is Gay-lussac’s Law In Simple Terms
What are some real-world examples of Gay-Lussac’s Law in action?
Besides hot air balloons, Gay-Lussac’s Law is also used in weather forecasting to predict changes in air pressure and volume, which can influence temperature and wind patterns. It’s also crucial in industrial processes like manufacturing and chemical reactions, where controlling temperature and volume is essential.
Does Gay-Lussac’s Law apply to liquids and solids?
No, Gay-Lussac’s Law specifically applies to gases. Liquids and solids have much stronger intermolecular forces, so their volume changes less dramatically with temperature.
How does Gay-Lussac’s Law relate to other gas laws?
Gay-Lussac’s Law is closely related to Boyle’s Law and Charles’ Law, which describe the relationship between pressure and volume, and volume and temperature, respectively. All three laws are combined into the ideal gas law, which provides a comprehensive understanding of the behavior of gases.