What is the law of conservation of energy sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This fundamental principle governs the universe, stating that energy cannot be created or destroyed, only transformed from one form to another. Throughout history, scientists have delved into the intricacies of energy, unraveling its secrets and unlocking its potential.
Imagine a swinging pendulum, a burning candle, or even the intricate workings of a power plant. These seemingly disparate phenomena are all interconnected by the law of conservation of energy. This law dictates that the total amount of energy within a closed system remains constant, even as it changes form. From the kinetic energy of motion to the potential energy stored within a battery, energy is constantly in flux, yet the total amount remains the same.
The Law of Conservation of Energy
The Law of Conservation of Energy is a fundamental principle in physics that states that energy cannot be created or destroyed, only transformed from one form to another. This means that the total amount of energy in a closed system remains constant over time. This principle is a cornerstone of modern physics and has profound implications for understanding the universe and our place within it.
Historical Context
The concept of energy conservation has roots in ancient philosophical ideas about the nature of the universe. However, the modern formulation of the law emerged in the 18th century, during the scientific revolution. This period saw significant advancements in mechanics, thermodynamics, and other fields, leading to a deeper understanding of energy and its transformations.
- 1747: The French mathematician and physicist, Emilie du Châtelet, translated Isaac Newton’s *Principia Mathematica* and introduced the concept of “vis viva” (living force), which is related to kinetic energy.
- 1842: The German physicist Julius Robert Mayer published his work on the equivalence of heat and mechanical work, which laid the foundation for the First Law of Thermodynamics.
- 1847: The English physicist James Prescott Joule conducted experiments that precisely measured the mechanical equivalent of heat, providing strong evidence for the conservation of energy.
- 1848: Hermann von Helmholtz formulated the principle of conservation of energy in a comprehensive manner, drawing on the work of Mayer and Joule. He stated that the total amount of energy in the universe is constant and that energy can only be transformed from one form to another.
Everyday Examples
The Law of Conservation of Energy applies to countless phenomena in our everyday lives. Here are some examples:
- Turning on a light bulb: Electrical energy is transformed into light and heat energy.
- Riding a bicycle: The energy you expend by pedaling is converted into kinetic energy of the bicycle and its rider.
- Burning wood in a fireplace: Chemical energy stored in the wood is released as heat and light energy.
- Charging a phone: Electrical energy is used to store chemical energy in the phone’s battery.
- A bouncing ball: Potential energy is converted to kinetic energy and back again as the ball rises and falls.
Forms of Energy
Energy exists in various forms, each representing a different way in which energy can be stored or transferred. Understanding these forms and their interconversions is crucial for comprehending the fundamental principles of energy and its applications in our daily lives.
Kinetic Energy
Kinetic energy is the energy possessed by an object due to its motion. The faster an object moves, the more kinetic energy it has. The relationship between kinetic energy (KE), mass (m), and velocity (v) is given by the following equation:
KE = 1/2 * mv2
For instance, a moving car possesses kinetic energy due to its speed. The faster the car moves, the greater its kinetic energy. This energy can be used to perform work, such as overcoming friction or accelerating the car.
Potential Energy
Potential energy is stored energy that an object possesses due to its position or configuration. It is the energy an object has because of its potential to do work. There are different types of potential energy, including:
Gravitational Potential Energy
Gravitational potential energy is the energy stored in an object due to its position relative to a gravitational field. The higher an object is lifted, the more gravitational potential energy it gains. The relationship between gravitational potential energy (PE), mass (m), gravitational acceleration (g), and height (h) is:
PE = mgh
For example, a book held above the ground possesses gravitational potential energy. When the book is released, this potential energy is converted into kinetic energy as the book falls.
Elastic Potential Energy
Elastic potential energy is the energy stored in an object due to its deformation, such as stretching or compressing. The more an object is stretched or compressed, the more elastic potential energy it stores. This energy is released when the object returns to its original shape.
For instance, a stretched rubber band possesses elastic potential energy. When the rubber band is released, this energy is converted into kinetic energy as the band snaps back to its original shape.
Chemical Potential Energy
Chemical potential energy is the energy stored in the bonds between atoms and molecules. This energy is released when chemical reactions occur, breaking or forming new bonds.
For example, the chemical potential energy stored in fuels like gasoline is released when the fuel is burned, converting the chemical energy into heat and light.
Thermal Energy
Thermal energy is the energy associated with the random motion of atoms and molecules within a substance. The higher the temperature of a substance, the more thermal energy it possesses.
For example, a hot cup of coffee possesses more thermal energy than a cold glass of water. This energy can be transferred to other objects through processes like conduction, convection, or radiation.
Electrical Energy
Electrical energy is the energy associated with the flow of electric charges. This energy can be generated by various sources, such as batteries, generators, and solar panels.
For instance, electrical energy powers our homes and appliances. It can be converted into other forms of energy, such as light energy in a light bulb or mechanical energy in an electric motor.
Nuclear Energy, What is the law of conservation of energy
Nuclear energy is the energy stored within the nucleus of an atom. This energy is released through nuclear reactions, such as fission and fusion.
For example, nuclear power plants use nuclear fission to generate electricity. Nuclear fusion is the process that powers the sun and other stars.
Energy Conversion
Energy can be converted from one form to another. For example, a hydroelectric dam converts the potential energy of water stored at a high elevation into kinetic energy as the water falls. This kinetic energy is then used to turn a turbine, which in turn generates electrical energy.
Other examples of energy conversion include:
* Solar panels convert light energy from the sun into electrical energy.
* A bicycle converts the mechanical energy of the rider’s legs into kinetic energy of the bicycle.
* A battery converts chemical potential energy into electrical energy.
* A stove converts chemical potential energy from burning fuel into thermal energy.
* A generator converts mechanical energy into electrical energy.
The law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another. This means that the total amount of energy in a closed system remains constant, even though the form of energy may change.
Energy Transformations and Transfers

Energy is not static; it constantly changes forms and moves between different systems. This dynamic aspect of energy is crucial to understanding its role in various natural phenomena and technological applications.
Energy Transformations
Energy transformations involve the conversion of one form of energy into another. These transformations are governed by the law of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another.
For instance, a hydroelectric power plant transforms the potential energy of water stored at a high elevation into kinetic energy as it falls, which is then converted into electrical energy by a turbine and generator. Similarly, a solar panel transforms light energy from the sun into electrical energy.
Energy Transfers
Energy transfers involve the movement of energy from one system to another. This transfer can occur through various mechanisms, including:
* Work: Work is done when a force acts over a distance, resulting in energy transfer. For example, lifting a weight involves doing work against gravity, transferring energy from your muscles to the weight.
* Heat: Heat transfer occurs due to temperature differences between systems. Heat flows from a hotter system to a colder one. For instance, a hot cup of coffee transfers heat to the surrounding air, causing the coffee to cool down.
Examples of Energy Transformations and Transfers
Swinging Pendulum
A swinging pendulum demonstrates both energy transformations and transfers. At its highest point, the pendulum possesses maximum potential energy and minimum kinetic energy. As it swings downwards, its potential energy is converted into kinetic energy, reaching maximum kinetic energy at the lowest point. This process reverses as the pendulum swings upwards, transforming kinetic energy back into potential energy.
Burning Candle
A burning candle exemplifies energy transformations and transfers in a chemical reaction. The candle’s wax stores chemical energy. When lit, the wax undergoes combustion, releasing heat and light energy. The heat energy transfers to the surrounding air, while the light energy radiates outward.
Other Examples
* Photosynthesis: Plants transform light energy from the sun into chemical energy stored in glucose molecules.
* Nuclear Power Plants: Nuclear power plants transform nuclear energy stored in uranium atoms into heat energy, which is then used to generate electricity.
* Fossil Fuel Combustion: The combustion of fossil fuels transforms chemical energy stored in hydrocarbons into heat energy, which is used for various purposes, including electricity generation and transportation.
Final Summary: What Is The Law Of Conservation Of Energy

The law of conservation of energy is a cornerstone of modern science, providing a framework for understanding the universe and its intricate processes. Its applications are far-reaching, influencing everything from the design of power plants to the development of energy-efficient technologies. While there are limitations and exceptions to this law, its fundamental principle remains a powerful tool for exploring the mysteries of energy and its role in our world.
Common Queries
Can energy truly be lost?
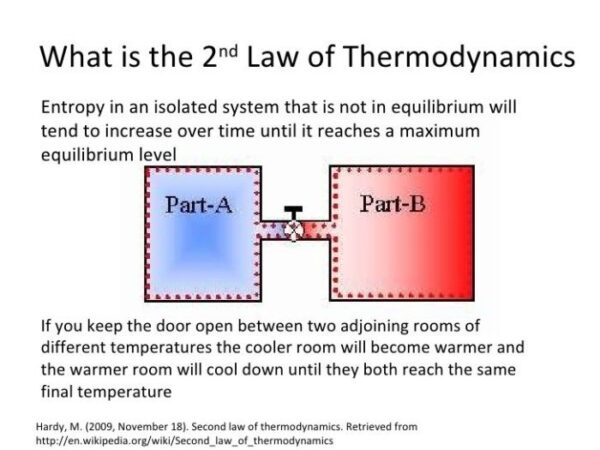
While energy cannot be destroyed, it can be dissipated or converted into less useful forms, such as heat. This process, known as entropy, explains why energy seems to be lost in some situations.
How does the law of conservation of energy apply to nuclear reactions?
The law of conservation of energy applies to nuclear reactions, but it’s important to consider the mass-energy equivalence. In nuclear reactions, some mass is converted into energy, and vice versa, but the total energy remains constant.
What are some examples of energy transformations in everyday life?
Turning on a light bulb transforms electrical energy into light and heat. A car engine converts chemical energy from fuel into mechanical energy to power the wheels. A hydroelectric dam transforms potential energy from water stored at a height into kinetic energy as it flows through the dam, generating electricity.