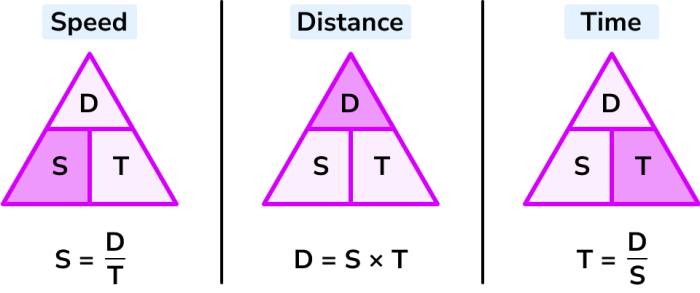
What is the Law of Multiple Proportions? This fundamental law of chemistry, discovered in the early 19th century, sheds light on how elements combine to form compounds. It reveals that when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.
Imagine two different compounds made from the same two elements. The Law of Multiple Proportions states that the ratio of the masses of one element in these compounds, when combined with a fixed mass of the other element, will always be a simple whole number ratio. This simple yet profound principle has revolutionized our understanding of chemical reactions and laid the foundation for modern chemistry.
Introduction to the Law of Multiple Proportions
The Law of Multiple Proportions is a fundamental concept in chemistry that describes the relationship between the masses of elements that combine to form different compounds. It states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers. This law helped to solidify the understanding of chemical reactions and provided a crucial step towards the development of the modern atomic theory.
Historical Context and Discovery
The Law of Multiple Proportions emerged during a period of significant scientific advancements in the late 18th and early 19th centuries. Chemists were actively trying to understand the nature of matter and the processes involved in chemical reactions. This pursuit led to the discovery of several important laws, including the Law of Conservation of Mass by Antoine Lavoisier and the Law of Definite Proportions by Joseph Proust.
The Law of Multiple Proportions was primarily formulated by John Dalton, an English chemist and physicist, in 1803. He was building upon the work of earlier scientists and was attempting to explain the observations made in chemical reactions. Dalton’s atomic theory, which he proposed around the same time, provided a theoretical framework for understanding the Law of Multiple Proportions.
Scientists Involved in its Discovery
Several scientists played key roles in the development and understanding of the Law of Multiple Proportions.
- John Dalton: Dalton is credited with formally stating the Law of Multiple Proportions. He meticulously studied the ratios of elements in different compounds and observed that the masses of one element combining with a fixed mass of another element were always in simple whole number ratios. This led him to propose his atomic theory, which explained the law and provided a foundation for understanding chemical reactions.
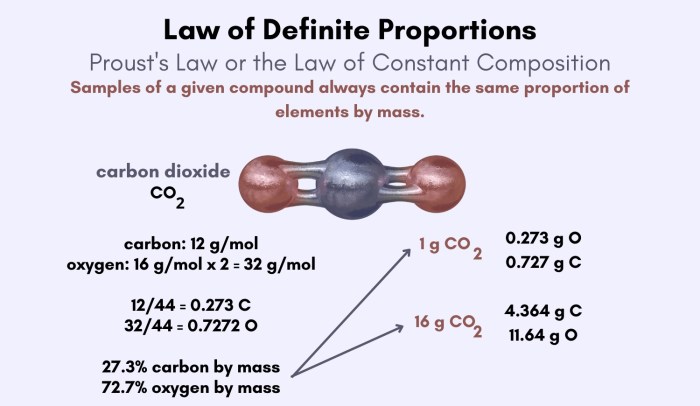
- Joseph Proust: Proust’s Law of Definite Proportions, which stated that a given chemical compound always contains the same elements in the same proportion by mass, provided a crucial foundation for Dalton’s work. Proust’s law established the consistency of chemical composition, paving the way for the discovery of the Law of Multiple Proportions.
- Antoine Lavoisier: Lavoisier’s Law of Conservation of Mass, which states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products, was also fundamental to the development of the Law of Multiple Proportions. Lavoisier’s work provided a framework for understanding mass relationships in chemical reactions, enabling scientists to accurately measure and analyze the proportions of elements in compounds.
Examples of its Application
The Law of Multiple Proportions was used to understand chemical reactions in the past by providing a way to predict the composition of compounds and to explain the relationships between different compounds.
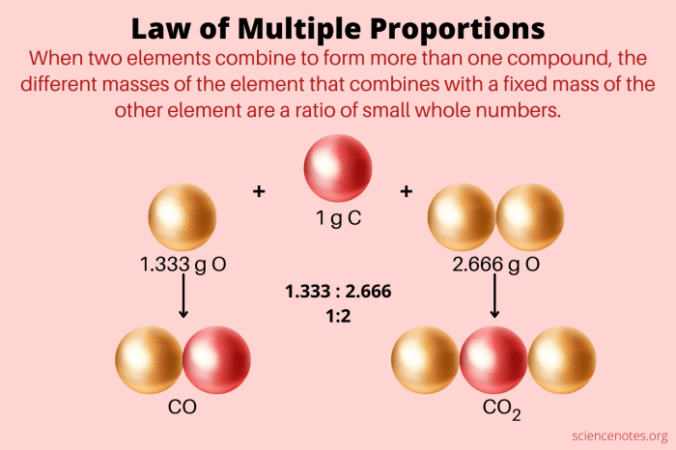
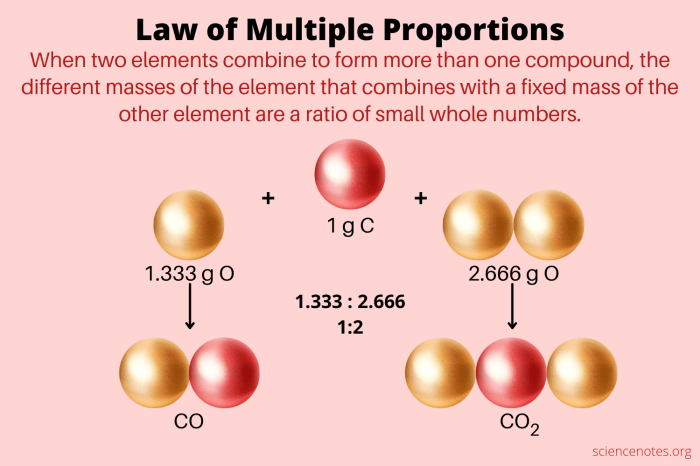
- Carbon Oxides: Dalton studied the compounds carbon monoxide (CO) and carbon dioxide (CO2). He found that for a fixed mass of carbon, the mass of oxygen in carbon dioxide was exactly twice the mass of oxygen in carbon monoxide. This observation supported the Law of Multiple Proportions, showing that the masses of oxygen combining with a fixed mass of carbon were in a simple whole number ratio (1:2).
- Nitrogen Oxides: Another example is the series of nitrogen oxides, such as nitrous oxide (N2O), nitric oxide (NO), nitrogen dioxide (NO2), and dinitrogen pentoxide (N2O5). By analyzing the masses of nitrogen and oxygen in these compounds, scientists could see that the masses of oxygen combining with a fixed mass of nitrogen were in simple whole number ratios, further supporting the Law of Multiple Proportions.
Defining the Law of Multiple Proportions

The Law of Multiple Proportions, a fundamental principle in chemistry, elucidates the way elements combine to form different compounds. It essentially states that when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.
Fixed Mass Ratios and Whole-Number Ratios
The Law of Multiple Proportions is based on the concept of fixed mass ratios, meaning that when elements combine to form a particular compound, they always do so in the same fixed ratio by mass. For example, in water (H2O), the mass ratio of hydrogen to oxygen is always 1:8, regardless of the amount of water.
Furthermore, the law highlights that when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers. Consider the example of carbon monoxide (CO) and carbon dioxide (CO2). In CO, the mass ratio of carbon to oxygen is 12:16, while in CO2, the mass ratio is 12:32. The ratio of the masses of oxygen that combine with a fixed mass of carbon in CO and CO2 is 16:32, which simplifies to 1:2, a simple whole-number ratio.
Significance of the Law of Multiple Proportions
The Law of Multiple Proportions has significant implications for understanding chemical reactions. It provides evidence for the existence of atoms and the concept of fixed proportions in chemical compounds. This law helps explain why elements combine in specific ratios to form compounds, leading to the development of the atomic theory and the concept of chemical formulas.
The Law of Multiple Proportions demonstrates that chemical reactions involve the combination of atoms in specific, whole-number ratios.
The law also plays a crucial role in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. By understanding the fixed mass ratios of elements in compounds, chemists can accurately predict the amounts of reactants and products involved in chemical reactions.
Examples of the Law of Multiple Proportions: What Is The Law Of Multiple Proportions
The Law of Multiple Proportions states that when two elements form more than one compound, the ratios of the masses of the second element that combine with a fixed mass of the first element are simple whole numbers. This law is a fundamental principle in chemistry that helps us understand the composition of chemical compounds.
Examples of Compounds with Multiple Proportions
To better understand the Law of Multiple Proportions, let’s examine some examples of compounds formed by two elements in different ratios. These examples illustrate how the masses of the elements in these compounds relate to each other in simple whole-number ratios.
| Compound Name | Element Ratio | Mass Ratio (Oxygen : Carbon) |
|---|---|---|
| Carbon monoxide (CO) | 1:1 | 1.33:1 |
| Carbon dioxide (CO2) | 1:2 | 2.66:1 |
The table shows that in carbon monoxide (CO), the mass ratio of oxygen to carbon is 1.33:1. In carbon dioxide (CO2), the mass ratio of oxygen to carbon is 2.66:1. Notice that the ratio of oxygen masses in CO2 to CO is 2:1, a simple whole-number ratio. This demonstrates the Law of Multiple Proportions, as the ratio of oxygen masses combining with a fixed mass of carbon (1 gram) is a simple whole number.
The Law of Multiple Proportions is essential for understanding the composition of chemical compounds. It allows us to predict the mass ratios of elements in different compounds formed by the same elements.
Applications of the Law of Multiple Proportions
The Law of Multiple Proportions is not just a theoretical concept; it has significant practical applications in chemistry. This law provides a framework for understanding how elements combine to form different compounds, and it plays a crucial role in determining the composition of these compounds.
Determining the Composition of Compounds, What is the law of multiple proportions
The Law of Multiple Proportions helps us determine the precise composition of compounds. When two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in a simple whole-number ratio. This principle allows us to deduce the relative proportions of elements in different compounds. For example, consider the two oxides of carbon, carbon monoxide (CO) and carbon dioxide (CO2). In CO, 12 grams of carbon combine with 16 grams of oxygen, while in CO2, 12 grams of carbon combine with 32 grams of oxygen. The ratio of oxygen masses in these two compounds is 16:32, which simplifies to 1:2, demonstrating the Law of Multiple Proportions.
Understanding the Formation of Different Compounds from the Same Elements
The Law of Multiple Proportions explains how different compounds can be formed from the same elements. This is possible because the elements can combine in different whole-number ratios. The law emphasizes that the formation of different compounds is not arbitrary but follows a specific pattern based on these ratios. For instance, carbon and oxygen can form carbon monoxide (CO) and carbon dioxide (CO2), where the ratio of oxygen atoms to carbon atoms differs. This difference in the ratio leads to the formation of distinct compounds with different properties.
Developing Chemical Formulas and Predicting the Properties of New Compounds
The Law of Multiple Proportions is fundamental in developing chemical formulas for compounds. By knowing the masses of elements combining in a compound, we can determine the simplest whole-number ratio of atoms, leading to the empirical formula. This empirical formula, along with other information like the molecular weight, can then be used to derive the molecular formula, which represents the actual number of atoms of each element in a molecule.
The Law of Multiple Proportions also aids in predicting the properties of new compounds. By analyzing the ratios of elements in known compounds and understanding the relationship between composition and properties, we can make informed predictions about the properties of new compounds. For example, if we know that the oxides of a certain metal exhibit specific properties, we can use the Law of Multiple Proportions to predict the properties of other oxides of the same metal based on the relative proportions of the elements.
Relationship to Other Laws of Chemistry
The Law of Multiple Proportions is intricately linked to other fundamental laws of chemistry, namely the Law of Conservation of Mass and the Law of Definite Proportions. Understanding these relationships provides a comprehensive view of how chemical reactions occur and how elements combine to form compounds.
The Law of Multiple Proportions builds upon the foundation laid by the Law of Definite Proportions and the Law of Conservation of Mass. These laws, together, provide a framework for understanding the quantitative relationships in chemical reactions.
Comparison with the Law of Conservation of Mass and the Law of Definite Proportions
The Law of Conservation of Mass states that in a closed system, the total mass of the reactants before a chemical reaction must equal the total mass of the products after the reaction. The Law of Definite Proportions states that a given chemical compound always contains the same elements in the same proportion by mass.
The Law of Multiple Proportions expands upon these principles by stating that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.
- The Law of Conservation of Mass emphasizes the conservation of matter during chemical reactions, while the Law of Definite Proportions focuses on the constant composition of a specific compound. The Law of Multiple Proportions goes a step further by revealing the relationships between the masses of elements in different compounds formed by the same elements.
- Consider the formation of carbon monoxide (CO) and carbon dioxide (CO2). The Law of Conservation of Mass states that the total mass of carbon and oxygen in the reactants will equal the total mass of carbon monoxide or carbon dioxide produced. The Law of Definite Proportions states that carbon monoxide will always have a fixed ratio of carbon and oxygen by mass, and similarly for carbon dioxide. The Law of Multiple Proportions tells us that the ratio of oxygen masses in carbon monoxide and carbon dioxide, when combined with a fixed mass of carbon, will be a simple whole number ratio (1:2 in this case).
Interconnectedness and Understanding of Chemical Reactions
These laws are interconnected and contribute to our understanding of chemical reactions in the following ways:
- The Law of Conservation of Mass provides the fundamental principle that matter is neither created nor destroyed in chemical reactions. This principle is essential for balancing chemical equations and understanding the stoichiometry of reactions.
- The Law of Definite Proportions establishes the constant composition of a specific compound. This law allows us to determine the empirical formula of a compound by analyzing its elemental composition.
- The Law of Multiple Proportions extends the concept of definite proportions to multiple compounds formed by the same elements. It helps us understand how elements can combine in different ratios to form different compounds, revealing the existence of different oxidation states and the versatility of chemical bonding.
Limitations and Exceptions
While these laws are fundamental to our understanding of chemistry, they do have some limitations and exceptions:
- The Law of Multiple Proportions applies primarily to compounds formed from two elements. In cases involving more than two elements, the ratios of masses may not be simple whole numbers.
- The law also assumes that the compounds are formed through simple chemical reactions. In complex reactions involving multiple steps or intermediates, the mass ratios may not always follow simple whole number relationships.
- The law does not apply to mixtures, where the composition can vary. Mixtures are not chemically bonded and do not have fixed proportions of their components.
Summary
The Law of Multiple Proportions, along with other fundamental laws of chemistry, provides a framework for understanding how elements combine to form compounds. It allows us to predict the composition of new compounds and delve deeper into the intricate world of chemical reactions. This law, discovered through meticulous observation and experimentation, stands as a testament to the power of scientific inquiry and its ability to unravel the secrets of the natural world.
Q&A
What are some real-world examples of the Law of Multiple Proportions?
One example is the oxides of nitrogen. Nitrogen and oxygen can form several different compounds, such as nitrous oxide (N₂O), nitric oxide (NO), nitrogen dioxide (NO₂), and dinitrogen pentoxide (N₂O₅). The Law of Multiple Proportions explains that the ratio of nitrogen to oxygen in these compounds will always be a simple whole number ratio.
How does the Law of Multiple Proportions relate to the Law of Definite Proportions?
The Law of Definite Proportions states that a given compound always contains the same elements in the same proportion by mass. The Law of Multiple Proportions expands on this concept by stating that when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.
What are the limitations of the Law of Multiple Proportions?
The Law of Multiple Proportions applies primarily to simple compounds formed by two elements. It may not hold true for complex compounds with multiple elements or for compounds where the elements can combine in different proportions depending on the conditions.