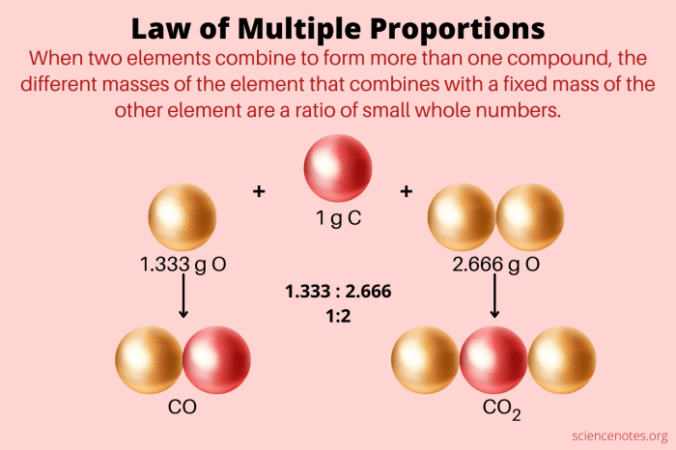
What law of conservation of mass – What is the Law of Conservation of Mass? This fundamental principle of chemistry states that in a closed system, the total mass of the reactants before a chemical reaction must equal the total mass of the products after the reaction. This concept, discovered by Antoine Lavoisier in the late 18th century, revolutionized our understanding of matter and its transformations.
The Law of Conservation of Mass is based on the idea that matter cannot be created or destroyed, only transformed. This means that during a chemical reaction, the atoms involved are merely rearranged, not lost or gained. The total mass of the substances involved in the reaction remains constant, even though their chemical composition may change.
Applications and Examples
The Law of Conservation of Mass has far-reaching implications and is a fundamental principle in many scientific disciplines and everyday life. It is applied in various fields, from chemistry and physics to cooking and baking, showcasing its importance in understanding and predicting how matter behaves.
Applications in Science, What law of conservation of mass
The Law of Conservation of Mass is a cornerstone principle in chemistry and physics, underpinning numerous concepts and calculations.
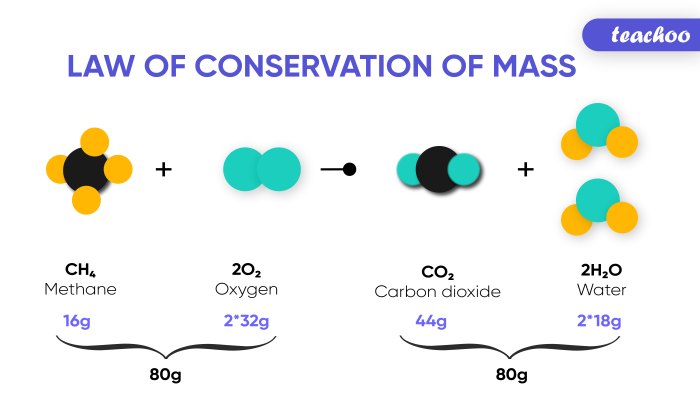
- Chemical Reactions: In chemical reactions, the total mass of the reactants must equal the total mass of the products. This principle is essential for balancing chemical equations and predicting the yield of reactions. For instance, in the reaction of hydrogen and oxygen to form water, the mass of the hydrogen and oxygen molecules before the reaction must equal the mass of the water molecules produced. This principle is also crucial for stoichiometry, which involves calculating the amounts of reactants and products in chemical reactions.
- Nuclear Reactions: While the Law of Conservation of Mass applies to most chemical reactions, it is slightly modified in nuclear reactions due to the conversion of mass into energy. In nuclear reactions, the total mass of the reactants may not be exactly equal to the total mass of the products due to the release or absorption of energy, as described by Einstein’s famous equation E=mc². However, the total mass-energy is conserved.
- Physics: In physics, the Law of Conservation of Mass is essential for understanding various phenomena, including momentum, energy, and collisions. For example, in a closed system, the total momentum of the system before and after a collision remains constant, even though individual objects may change their momentum. This principle is crucial for analyzing the behavior of particles and systems in various contexts.
Applications in Everyday Life
The Law of Conservation of Mass is evident in many everyday activities, from cooking to baking.
- Cooking: When cooking, the total mass of the ingredients before cooking must equal the total mass of the dish after cooking. This principle is especially important when baking, where precise measurements are crucial for successful outcomes. For example, when baking a cake, the mass of the flour, sugar, eggs, and other ingredients must equal the mass of the finished cake, minus any moisture lost during baking.
- Recycling: The Law of Conservation of Mass is fundamental to recycling. When materials are recycled, the total mass of the original material must equal the total mass of the recycled product, even though the material may be transformed into a new form. This principle ensures that resources are not lost during the recycling process.
- Combustion: The burning of fuel, such as wood or gasoline, is a chemical reaction that releases energy. The Law of Conservation of Mass applies here, meaning that the total mass of the fuel and oxygen before combustion must equal the total mass of the products, including ash, carbon dioxide, and water vapor. The difference in mass is due to the energy released during the reaction.
Illustrations and Visual Representations
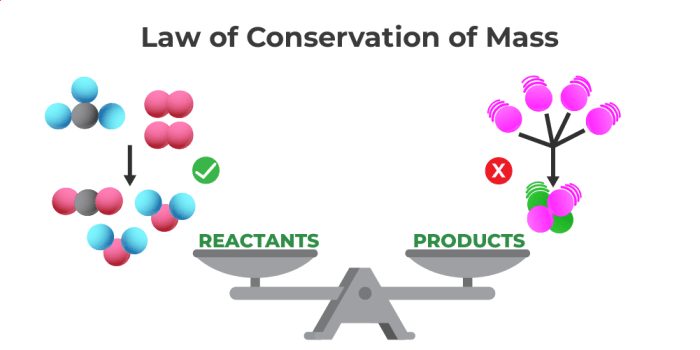
Visual representations can be very helpful in understanding and illustrating the Law of Conservation of Mass. They provide a clear and concise way to visualize the concept of mass being neither created nor destroyed in a chemical reaction.
Visual Representation of the Law of Conservation of Mass
A common visual representation of the Law of Conservation of Mass is a diagram depicting a chemical reaction with reactants and products. The diagram would show the mass of the reactants before the reaction and the mass of the products after the reaction. The total mass of the reactants and products would be equal, demonstrating that mass is conserved.
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 → 2H2O
The diagram would show two molecules of hydrogen gas (H2) and one molecule of oxygen gas (O2) as reactants. The products would be two molecules of water (H2O). The total mass of the reactants would be equal to the total mass of the products, illustrating the conservation of mass.
The key elements of the visual representation include:
- Reactants: The substances that are present at the beginning of the reaction. In the example above, the reactants are hydrogen gas (H2) and oxygen gas (O2).
- Products: The substances that are formed as a result of the reaction. In the example above, the product is water (H2O).
- Mass: The amount of matter in a substance. The diagram would show the mass of each reactant and product, demonstrating that the total mass remains constant throughout the reaction.
- Arrows: The arrows in the diagram indicate the direction of the reaction. The arrow pointing to the right indicates that the reactants are reacting to form the products.
This type of visual representation helps to illustrate the Law of Conservation of Mass by showing that the total mass of the reactants before the reaction is equal to the total mass of the products after the reaction. This visual representation provides a clear and concise way to understand the concept of mass being conserved in chemical reactions.
Implications and Importance

The Law of Conservation of Mass is a fundamental principle in chemistry and has profound implications for understanding the nature of matter and its transformations. It provides a crucial framework for comprehending chemical reactions and lays the foundation for numerous scientific advancements.
Impact on Understanding Chemical Reactions
The Law of Conservation of Mass is a cornerstone of chemical reactions. It states that in a closed system, the total mass of the reactants before a chemical reaction must equal the total mass of the products after the reaction. This principle is essential for balancing chemical equations, ensuring that the number of atoms of each element remains constant throughout the reaction. By understanding this principle, chemists can accurately predict the amounts of reactants and products involved in a reaction, enabling them to design and optimize chemical processes.
End of Discussion: What Law Of Conservation Of Mass
The Law of Conservation of Mass is a cornerstone of chemistry and physics, providing a foundational understanding of matter and its behavior. Its applications extend far beyond the laboratory, influencing our understanding of everything from cooking and baking to industrial processes and environmental concerns. By recognizing that matter is conserved, we gain a deeper appreciation for the intricate dance of atoms and molecules that shapes our world.
FAQ Summary
What are some everyday examples of the Law of Conservation of Mass?
Burning a piece of wood is a good example. The wood appears to disappear, but the mass of the wood is actually converted into ash, smoke, and gases. The total mass of the products (ash, smoke, and gases) will equal the mass of the original piece of wood.
Does the Law of Conservation of Mass apply to nuclear reactions?
No, the Law of Conservation of Mass does not strictly apply to nuclear reactions. In nuclear reactions, a small amount of mass can be converted into energy, as described by Einstein’s famous equation E=mc².