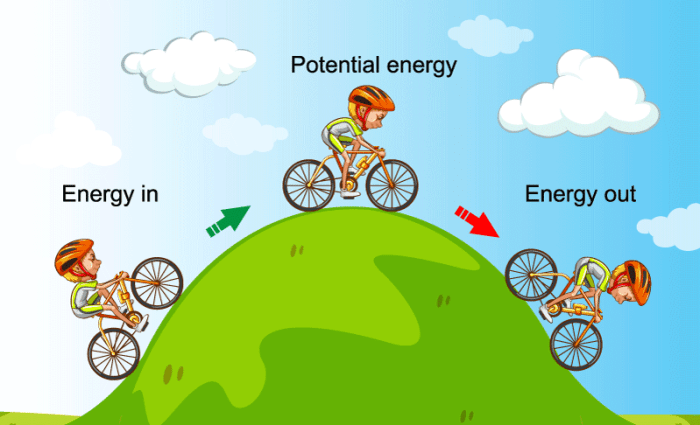
Which law is energy cannot be created or destroyed – The Law of Conservation of Energy: Energy Cannot Be Created or Destroyed – this fundamental principle governs the universe, dictating that energy can neither be created nor destroyed, but only transformed from one form to another. Imagine a child on a swing. As they push off the ground, their potential energy is converted into kinetic energy, propelling them upwards. At the peak of their swing, the kinetic energy transforms back into potential energy, ready for the cycle to repeat. This simple example illustrates the law’s core principle: energy is constantly changing forms, but its total amount remains constant.
This law, deeply rooted in physics, has profound implications for our understanding of the universe. It provides a framework for analyzing energy transformations in everyday life, from burning fuel for heat to generating electricity from wind or solar power. Its applications extend across various fields, including engineering, physics, and chemistry, guiding the development of efficient technologies and advancing our knowledge of the natural world.
The Law of Conservation of Energy
The law of conservation of energy is a fundamental principle in physics that states that energy cannot be created or destroyed, only transformed from one form to another. This means that the total amount of energy in a closed system remains constant over time, even as it changes form.
The Fundamental Principle
The law of conservation of energy is based on the idea that energy is a fundamental property of the universe, and it cannot be created or destroyed. It can only be transformed from one form to another, such as from potential energy to kinetic energy or from chemical energy to heat energy. This principle is essential for understanding how the universe works, and it has many important applications in science and technology.
Real-World Examples of Energy Transformation
There are countless examples of energy transformation in the world around us. Here are a few:
- A hydroelectric dam: The potential energy of water stored at a high elevation is transformed into kinetic energy as the water flows down and turns a turbine. This kinetic energy is then converted into electrical energy by the generator.
- A burning candle: The chemical energy stored in the wax is transformed into heat and light energy as the candle burns.
- A solar panel: Light energy from the sun is transformed into electrical energy in a solar panel.
Historical Development of the Law
The concept of conservation of energy has a long and fascinating history, with contributions from many scientists over centuries. Here are some key milestones:
- 17th Century: René Descartes and Gottfried Wilhelm Leibniz laid the groundwork by proposing that a quantity called “vis viva” (living force), later identified as kinetic energy, was conserved in collisions.
- 18th Century: Émilie du Châtelet, a prominent physicist and mathematician, translated and critiqued Isaac Newton’s work, contributing to the understanding of energy conservation.
- 19th Century: Julius Robert Mayer, James Prescott Joule, and Hermann von Helmholtz independently formulated the law of conservation of energy. Mayer proposed the equivalence of heat and mechanical work, while Joule established the mechanical equivalent of heat through his famous experiments. Helmholtz provided a more comprehensive formulation of the law, encompassing all forms of energy.
The law of conservation of energy is one of the most fundamental and important principles in physics. It is a cornerstone of our understanding of the universe and has countless applications in science and technology.
Energy Transformations and Examples
The Law of Conservation of Energy states that energy cannot be created or destroyed, but it can be transformed from one form to another. This means that energy is constantly changing its form, moving from one type to another. These transformations occur in countless ways, from the simple act of burning fuel to the complex processes that power our world.
Examples of Energy Transformations, Which law is energy cannot be created or destroyed
Energy transformations are a fundamental aspect of our daily lives. They are the driving force behind many of the processes we rely on, from heating our homes to powering our devices. Here are some examples of energy transformations:
- Burning fuel for heat: When we burn fuel, such as wood or natural gas, the chemical energy stored in the fuel is transformed into thermal energy (heat). This heat can then be used to warm our homes, cook our food, or generate electricity.
- Generating electricity from wind or solar power: Wind turbines convert the kinetic energy of wind into mechanical energy, which is then used to generate electricity. Solar panels convert the radiant energy of sunlight into electrical energy. These are examples of energy transformations from one form to another.
- Charging a battery: When we charge a battery, we are converting electrical energy into chemical energy. This chemical energy is stored within the battery and can be released later to power devices.
Forms of Energy and Their Transformations
The following table illustrates various forms of energy and their transformations:
| Form of Energy | Description | Transformation Examples |
|---|---|---|
| Kinetic Energy | Energy of motion | – A moving car possesses kinetic energy. – Wind turbines convert kinetic energy of wind into mechanical energy. |
| Potential Energy | Stored energy due to position or state | – A book on a shelf has potential energy due to its height. – A stretched rubber band stores potential energy. |
| Chemical Energy | Energy stored in the bonds of molecules | – Burning fuel releases chemical energy as heat. – Batteries store chemical energy and release it as electrical energy. |
| Thermal Energy | Energy associated with the temperature of a substance | – Heat from the sun is thermal energy. – Burning fuel converts chemical energy into thermal energy. |
| Radiant Energy | Energy that travels in waves, such as light and heat | – Sunlight is radiant energy. – Solar panels convert radiant energy into electrical energy. |
| Electrical Energy | Energy associated with the flow of electric charges | – Power plants generate electrical energy. – Electrical appliances convert electrical energy into other forms, such as heat, light, or motion. |
| Nuclear Energy | Energy stored in the nucleus of an atom | – Nuclear power plants convert nuclear energy into electrical energy. – Nuclear weapons release a tremendous amount of nuclear energy. |
Applications of the Law of Conservation of Energy
The law of conservation of energy is a fundamental principle in physics and has numerous applications in various fields. It is applied to understand and predict energy transformations in systems and to design efficient technologies.
Engineering
The law of conservation of energy plays a crucial role in engineering, particularly in the design and analysis of energy systems. Engineers apply this principle to optimize energy efficiency and minimize energy losses in various applications.
- Designing Efficient Engines: Internal combustion engines are designed based on the principle of conservation of energy. The chemical energy stored in fuel is converted into mechanical energy to power vehicles. Engineers strive to maximize the conversion efficiency by minimizing energy losses due to friction, heat dissipation, and other factors. This ensures that the engine operates efficiently and consumes less fuel.
- Power Generation and Distribution: The law of conservation of energy is essential in power generation and distribution systems. Power plants convert energy from various sources, such as fossil fuels, nuclear reactions, or renewable sources, into electricity. The energy generated is then distributed to consumers through a network of transmission lines and distribution grids. The law of conservation of energy ensures that the total energy input equals the total energy output, accounting for losses during transmission and distribution.
- Thermal Engineering: Thermal engineering involves the study and application of heat transfer. The law of conservation of energy is used to analyze heat flow in systems and to design efficient heat exchangers, insulation systems, and other thermal devices. For example, in a refrigerator, the law of conservation of energy is used to ensure that the cooling system effectively removes heat from the refrigerator compartment while minimizing energy consumption.
Physics
The law of conservation of energy is a fundamental principle in physics, used to analyze energy flow in systems and to understand the behavior of matter.
- Analyzing Energy Flow in Systems: Physicists use the law of conservation of energy to analyze energy transformations in various systems, such as mechanical systems, electrical circuits, and thermodynamic systems. By tracking the energy flow, they can determine the efficiency of energy conversion processes and identify potential energy losses. This knowledge is crucial for understanding and predicting the behavior of these systems.
- Understanding Fundamental Interactions: The law of conservation of energy is essential for understanding fundamental interactions in physics, such as the interaction between particles and fields. For example, in particle physics, the law of conservation of energy is used to analyze collisions between particles and to understand the creation and annihilation of particles. It helps physicists to understand the fundamental building blocks of matter and the forces that govern their interactions.
- Developing New Technologies: The law of conservation of energy is used to develop new technologies, such as solar cells, batteries, and fuel cells. These technologies aim to harness and store energy from various sources, such as sunlight, chemical reactions, and nuclear reactions. The law of conservation of energy ensures that the energy input equals the energy output in these systems, allowing for efficient energy conversion and storage.
Chemistry
The law of conservation of energy is fundamental in chemistry, used to understand chemical reactions and to predict the energy changes involved.
- Understanding Chemical Reactions: The law of conservation of energy is used to understand the energy changes that occur during chemical reactions. Chemical reactions involve the breaking and formation of chemical bonds, which release or absorb energy. The law of conservation of energy ensures that the total energy of the reactants equals the total energy of the products, accounting for any energy released or absorbed during the reaction. This allows chemists to predict the energy changes involved in reactions and to design reactions that release or absorb specific amounts of energy.
- Thermochemistry: Thermochemistry is the study of energy changes in chemical reactions. The law of conservation of energy is used to calculate the enthalpy change (heat change) of reactions and to determine the amount of heat released or absorbed during a reaction. This information is crucial for understanding the feasibility of reactions and for designing processes that utilize or control heat energy.
- Chemical Kinetics: Chemical kinetics is the study of the rates and mechanisms of chemical reactions. The law of conservation of energy is used to analyze the energy barriers that must be overcome for reactions to occur. This knowledge is essential for understanding the factors that influence reaction rates and for developing catalysts that can speed up reactions.
Applications in Various Fields
| Field | Application | Relevance |
|---|---|---|
| Engineering | Designing efficient engines | Maximizes energy conversion efficiency and minimizes fuel consumption. |
| Power generation and distribution | Ensures energy conservation in power systems and minimizes losses during transmission and distribution. | |
| Thermal engineering | Analyzes heat flow and designs efficient heat exchangers and insulation systems. | |
| Physics | Analyzing energy flow in systems | Determines energy conversion efficiency and identifies potential energy losses. |
| Understanding fundamental interactions | Explains the behavior of particles and forces in particle physics. | |
| Developing new technologies | Ensures efficient energy conversion and storage in solar cells, batteries, and fuel cells. | |
| Chemistry | Understanding chemical reactions | Predicts energy changes in reactions and designs reactions that release or absorb specific amounts of energy. |
| Thermochemistry | Calculates enthalpy change of reactions and determines heat released or absorbed. | |
| Chemical kinetics | Analyzes energy barriers in reactions and develops catalysts to speed up reactions. |
Ending Remarks
The Law of Conservation of Energy stands as a cornerstone of scientific understanding, providing a powerful lens through which we can analyze the intricate workings of the universe. It reminds us that energy is not a limitless resource, but rather a precious commodity that must be managed wisely. By understanding and applying this law, we can strive for a future where energy is used efficiently and sustainably, minimizing our impact on the environment and ensuring a brighter future for generations to come.
Expert Answers: Which Law Is Energy Cannot Be Created Or Destroyed
What are some common misconceptions about the Law of Conservation of Energy?
One common misconception is that energy can be destroyed. While energy can be transformed, it cannot be destroyed. Another misconception is that perpetual motion machines are possible. These machines would violate the Law of Conservation of Energy, as they would create energy from nothing.
How does the Law of Conservation of Energy relate to the concept of entropy?
While the Law of Conservation of Energy states that energy cannot be created or destroyed, the concept of entropy states that the total entropy of an isolated system can never decrease over time. This means that energy transformations always result in a decrease in usable energy and an increase in unusable energy, such as heat.
What are some examples of energy conservation in everyday life?
Some examples of energy conservation in everyday life include turning off lights when leaving a room, using energy-efficient appliances, and walking or cycling instead of driving short distances.